Preclinical development of monoclonal antibodies: considerations for the use of non-human primates
- PMID: 20065651
- PMCID: PMC2759500
- DOI: 10.4161/mabs.1.5.9676
Preclinical development of monoclonal antibodies: considerations for the use of non-human primates
Abstract
The development of mAbs remains high on the therapeutic agenda for the majority of pharmaceutical and biotechnology companies. Often, the only relevant species for preclinical safety assessment of mAbs are non-human primates (NHPs), and this raises important scientific, ethical and economic issues. To investigate evidence-based opportunities to minimize the use of NHPs, an expert working group with representatives from leading pharmaceutical and biotechnology companies, contract research organizations and institutes from Europe and the USA, has shared and analyzed data on mAbs for a range of therapeutic areas. This information has been applied to hypothetical examples to recommend scientifically appropriate development pathways and study designs for a variety of potential mAbs. The addendum of ICHS6 provides a timely opportunity for the scientific and regulatory community to embrace strategies which minimize primate use and increase efficiency of mAb development.
Figures

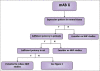
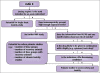
Similar articles
-
Immunogenicity of mAbs in non-human primates during nonclinical safety assessment.MAbs. 2013 Sep-Oct;5(5):810-6. doi: 10.4161/mabs.25234. Epub 2013 Jun 6. MAbs. 2013. PMID: 23924803 Free PMC article.
-
Considerations regarding nonhuman primate use in safety assessment of biopharmaceuticals.Int J Toxicol. 2011 Oct;30(5):583-90. doi: 10.1177/1091581811415875. Int J Toxicol. 2011. PMID: 22013138 Review.
-
Preclinical safety evaluation of monoclonal antibodies.Handb Exp Pharmacol. 2008;(181):19-44. doi: 10.1007/978-3-540-73259-4_2. Handb Exp Pharmacol. 2008. PMID: 18071940 Review.
-
Safety testing of monoclonal antibodies in non-human primates: Case studies highlighting their impact on human risk assessment.MAbs. 2018 Jan;10(1):1-17. doi: 10.1080/19420862.2017.1389364. Epub 2017 Oct 26. MAbs. 2018. PMID: 28991509 Free PMC article. Review.
-
Preclinical safety testing of monoclonal antibodies: the significance of species relevance.Nat Rev Drug Discov. 2007 Feb;6(2):120-6. doi: 10.1038/nrd2242. Nat Rev Drug Discov. 2007. PMID: 17268483 Review.
Cited by
-
Immunogenicity of mAbs in non-human primates during nonclinical safety assessment.MAbs. 2013 Sep-Oct;5(5):810-6. doi: 10.4161/mabs.25234. Epub 2013 Jun 6. MAbs. 2013. PMID: 23924803 Free PMC article.
-
Waiving in vivo studies for monoclonal antibody biosimilar development: National and global challenges.MAbs. 2016;8(3):427-35. doi: 10.1080/19420862.2016.1145331. MAbs. 2016. PMID: 26854177 Free PMC article. Review.
-
Opportunities to Apply the 3Rs in Safety Assessment Programs.ILAR J. 2016 Dec;57(2):234-245. doi: 10.1093/ilar/ilw024. ILAR J. 2016. PMID: 28053076 Free PMC article.
-
Integrated continuous bioprocessing: Economic, operational, and environmental feasibility for clinical and commercial antibody manufacture.Biotechnol Prog. 2017 Jul;33(4):854-866. doi: 10.1002/btpr.2492. Epub 2017 Jun 2. Biotechnol Prog. 2017. PMID: 28480535 Free PMC article.
-
Challenges and approaches for the development of safer immunomodulatory biologics.Nat Rev Drug Discov. 2013 Apr;12(4):306-24. doi: 10.1038/nrd3974. Nat Rev Drug Discov. 2013. PMID: 23535934 Free PMC article. Review.
References
-
- DataMonitor, author. Monoclonal Antibodies report part II: Companies—holding mAbs in portfolio promises protection against the looming 2011–2012 patent cliff. 2007.
-
- Reichert JM. Monoclonal antibodies as innovative therapies. Curr Pharm Biotechnol. 2008;9:423–430. - PubMed
-
- Reichert JM, Rosenweig CJ, Faden L, Dewitz MC. Monoclonal antibodies successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. - PubMed
-
- Wordell CJ. Biotechnology update. Hosp Pharm. 1991;26:897–900.
-
- OKT3: Ortho Pharmaceutical Corporation, author. Summary basis of approval of Muromonab-CD3. Freedom of Information Services FDA. 1986.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
