Affinity maturation and characterization of a human monoclonal antibody against HIV-1 gp41
- PMID: 20065653
- PMCID: PMC2759496
- DOI: 10.4161/mabs.1.5.9214
Affinity maturation and characterization of a human monoclonal antibody against HIV-1 gp41
Abstract
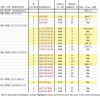
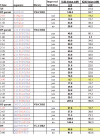
The human D5 monoclonal antibody binds to the highly conserved hydrophobic pocket on the N-terminal heptad repeat (NHR) trimer of HIV-1 gp41 and exhibits modest yet relatively broad neutralization activity. Both binding and neutralization depend on residues in the complementarity determining regions (CDRs) of the D5 IgG variable domains on heavy chain (VH) and light chain (VL). In an effort to increase neutralization activity to a wider range of HIV-1 strains, we have affinity matured the parental D5 scFv by randomizing selected residues in 5 of its 6 CDRs. The resulting scFv variants derived from four different CDR changes showed enhanced binding affinities to gp41 NHR mimetic (5-helix) which correlated to improved neutralization potencies by up to 8-fold. However, when converted to IgG1s, these D5 variants had up to a 12-fold reduction in neutralization potency over their corresponding scFvs despite their slightly enhanced in vitro binding affinities. Remarkably, D5 variant IgG1s bearing residue changes in CDRs that interact with epitope residues N-terminal to the hydrophobic pocket (such as VH CDR3 and VL CDR3) retained more neutralization potency than those containing residue changes in pocket-interacting CDRs (such as VH CDR2). These results provide compelling evidence for the existence of a steric block to an IgG that extends to the gp41 NHR hydrophobic pocket region, and can be a useful guide for developing therapeutic antibodies and vaccines circumventing this block.
Figures

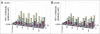




Similar articles
-
Crystal structure and size-dependent neutralization properties of HK20, a human monoclonal antibody binding to the highly conserved heptad repeat 1 of gp41.PLoS Pathog. 2010 Nov 18;6(11):e1001195. doi: 10.1371/journal.ppat.1001195. PLoS Pathog. 2010. PMID: 21124990 Free PMC article.
-
A Derivative of the D5 Monoclonal Antibody That Targets the gp41 N-Heptad Repeat of HIV-1 with Broad Tier-2-Neutralizing Activity.J Virol. 2021 Jul 12;95(15):e0235020. doi: 10.1128/JVI.02350-20. Epub 2021 Jul 12. J Virol. 2021. PMID: 33980592 Free PMC article.
-
Affinity maturation by targeted diversification of the CDR-H2 loop of a monoclonal Fab derived from a synthetic naïve human antibody library and directed against the internal trimeric coiled-coil of gp41 yields a set of Fabs with improved HIV-1 neutralization potency and breadth.Virology. 2009 Oct 10;393(1):112-9. doi: 10.1016/j.virol.2009.07.019. Epub 2009 Aug 19. Virology. 2009. PMID: 19695655 Free PMC article.
-
Short communication: In vitro synergy between peptides or neutralizing antibodies targeting the N- and C-terminal heptad repeats of HIV Type 1 gp41.AIDS Res Hum Retroviruses. 2008 Dec;24(12):1537-44. doi: 10.1089/aid.2008.0129. AIDS Res Hum Retroviruses. 2008. PMID: 19102685 Review.
-
Antigp41 membrane proximal external region antibodies and the art of using the membrane for neutralization.Curr Opin HIV AIDS. 2017 May;12(3):250-256. doi: 10.1097/COH.0000000000000364. Curr Opin HIV AIDS. 2017. PMID: 28422789 Review.
Cited by
-
Crystal structure and size-dependent neutralization properties of HK20, a human monoclonal antibody binding to the highly conserved heptad repeat 1 of gp41.PLoS Pathog. 2010 Nov 18;6(11):e1001195. doi: 10.1371/journal.ppat.1001195. PLoS Pathog. 2010. PMID: 21124990 Free PMC article.
-
A Derivative of the D5 Monoclonal Antibody That Targets the gp41 N-Heptad Repeat of HIV-1 with Broad Tier-2-Neutralizing Activity.J Virol. 2021 Jul 12;95(15):e0235020. doi: 10.1128/JVI.02350-20. Epub 2021 Jul 12. J Virol. 2021. PMID: 33980592 Free PMC article.
-
Side chain requirements for affinity and specificity in D5, an HIV-1 antibody derived from the VH1-69 germline segment.BMC Biochem. 2013 Apr 8;14:9. doi: 10.1186/1471-2091-14-9. BMC Biochem. 2013. PMID: 23566198 Free PMC article.
-
Novel neutralising antibodies targeting the N-terminal helical region of the transmembrane envelope protein p15E of the porcine endogenous retrovirus (PERV).Immunol Res. 2014 Jan;58(1):9-19. doi: 10.1007/s12026-013-8430-y. Immunol Res. 2014. PMID: 23729215
-
Bioinformatics-led design of single-chain antibody molecules targeting DNA sequences for retinoblastoma.Int J Ophthalmol. 2011;4(1):8-13. doi: 10.3980/j.issn.2222-3959.2011.01.02. Epub 2011 Feb 18. Int J Ophthalmol. 2011. PMID: 22553599 Free PMC article.
References
-
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–236. - PubMed
-
- Schiavone M, Quinto I, Scala G. Perspectives for a protective HIV-1 vaccine. Adv Pharmacol. 2008;56:423–452. - PubMed
-
- Root MJ, Steger HK. HIV-1 gp41 as a target for viral entry inhibition. Curr Pharm Des. 2004;10:1805–1825. - PubMed
-
- Zhu P, Liu J, Bess J, Jr, Chertova E, Lifson JD, Grise H, et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441:847–852. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
