A dual-targeting PDGFRbeta/VEGF-A molecule assembled from stable antibody fragments demonstrates anti-angiogenic activity in vitro and in vivo
- PMID: 20065654
- PMCID: PMC2828575
- DOI: 10.4161/mabs.2.1.10498
A dual-targeting PDGFRbeta/VEGF-A molecule assembled from stable antibody fragments demonstrates anti-angiogenic activity in vitro and in vivo
Abstract
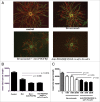
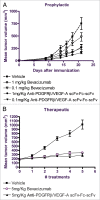
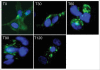
Targeting angiogenesis is a promising approach to the treatment of solid tumors and age-related macular degeneration (AMD). Inhibition of vascularization has been validated by the successful marketing of monoclonal antibodies (mAbs) that target specific growth factors or their receptors, but there is considerable room for improvement in existing therapies. Combination of mAbs targeting both the VEGF and PDGF pathways has the potential to increase the efficacy of anti-angiogenic therapy without the accompanying toxicities of tyrosine kinase inhibitors and the inability to combine efficiently with traditional chemotherapeutics. However, development costs and regulatory issues have limited the use of combinatorial approaches for the generation of more efficacious treatments. The concept of mediating disease pathology by targeting two antigens with one therapeutic was proposed over two decades ago. While mAbs are particularly suitable candidates for a dual-targeting approach, engineering bispecificity into one molecule can be difficult due to issues with expression and stability, which play a significant role in manufacturability. Here, we address these issues upstream in the process of developing a bispecific antibody (bsAb). Single-chain antibody fragments (scFvs) targeting PDGFRbeta and VEGF-A were selected for superior stability. The scFvs were fused to both termini of human Fc to generate a bispecific, tetravalent molecule. The resulting molecule displays potent activity, binds both targets simultaneously, and is stable in serum. The assembly of a bsAb using stable monomeric units allowed development of an anti-PDGFRB/VEGF-A antibody capable of attenuating angiogenesis through two distinct pathways and represents an efficient method for rapid engineering of dual-targeting molecules.
Figures


Similar articles
-
A bi-functional antibody-receptor domain fusion protein simultaneously targeting IGF-IR and VEGF for degradation.MAbs. 2015;7(5):931-45. doi: 10.1080/19420862.2015.1055442. MAbs. 2015. PMID: 26073904 Free PMC article.
-
A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity.MAbs. 2011 May-Jun;3(3):273-88. doi: 10.4161/mabs.3.3.15188. Epub 2011 May 1. MAbs. 2011. PMID: 21393993 Free PMC article.
-
Generation and characterization of ABBV642, a dual variable domain immunoglobulin molecule (DVD-Ig) that potently neutralizes VEGF and PDGF-BB and is designed for the treatment of exudative age-related macular degeneration.MAbs. 2017 Feb/Mar;9(2):269-284. doi: 10.1080/19420862.2016.1268305. MAbs. 2017. PMID: 27929753 Free PMC article.
-
Therapeutic anti-VEGF antibodies.Handb Exp Pharmacol. 2008;(181):131-50. doi: 10.1007/978-3-540-73259-4_6. Handb Exp Pharmacol. 2008. PMID: 18071944 Review.
-
Therapeutic bispecific antibodies: The selection of stable single-chain fragments to overcome engineering obstacles.IDrugs. 2010 Aug;13(8):543-9. IDrugs. 2010. PMID: 20721825 Review.
Cited by
-
A bi-functional antibody-receptor domain fusion protein simultaneously targeting IGF-IR and VEGF for degradation.MAbs. 2015;7(5):931-45. doi: 10.1080/19420862.2015.1055442. MAbs. 2015. PMID: 26073904 Free PMC article.
-
Cancer-associated fibroblasts as therapeutic targets for cancer: advances, challenges, and future prospects.J Biomed Sci. 2025 Jan 9;32(1):7. doi: 10.1186/s12929-024-01099-2. J Biomed Sci. 2025. PMID: 39780187 Free PMC article. Review.
-
Sorafenib-loaded nanostructured lipid carriers for topical ocular therapy of corneal neovascularization: development, in-vitro and in vivo study.Drug Deliv. 2022 Dec;29(1):837-855. doi: 10.1080/10717544.2022.2048134. Drug Deliv. 2022. PMID: 35277107 Free PMC article.
-
CDR-restricted engineering of native human scFvs creates highly stable and soluble bifunctional antibodies for subcutaneous delivery.MAbs. 2013 Nov-Dec;5(6):882-95. doi: 10.4161/mabs.26201. Epub 2013 Aug 21. MAbs. 2013. PMID: 23995618 Free PMC article.
-
Fit for the Eye: Aptamers in Ocular Disorders.Nucleic Acid Ther. 2016 Jun;26(3):127-46. doi: 10.1089/nat.2015.0573. Epub 2016 Jan 12. Nucleic Acid Ther. 2016. PMID: 26757406 Free PMC article. Review.
References
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. - PubMed
-
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Disc. 2007;6:273–286. - PubMed
-
- Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med. 2007;58:491–504. - PubMed
-
- Gaur P, Bose D, Samuel S, Ellis LM. Targeting tumor angiogenesis. Semin Oncol. 2009;36:12–19. - PubMed
-
- Shojaei F, Ferrara N. Antiangiogenic therapy for cancer: an update. Cancer J. 2007;13:345–348. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
