Human peritoneal macrophages from ascitic fluid can be infected by a broad range of HIV-1 isolates
- PMID: 20065862
- PMCID: PMC2840202
- DOI: 10.1097/QAI.0b013e3181ca3401
Human peritoneal macrophages from ascitic fluid can be infected by a broad range of HIV-1 isolates
Abstract
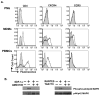
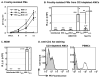
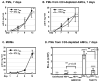
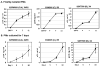
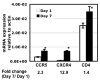
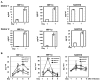
Macrophages are major HIV target cells. They support both productive and latent HIV-1 infection. Susceptibility of primary macrophages to HIV depends on the anatomical location and activation state of the cells. We demonstrate that peritoneal macrophages (PMs) are abundant in ascitic fluid of patients with liver cirrhosis and are susceptible to HIV-1 infection. PMs expressed CD68, a differentiation marker, exhibited phagocytic activity, and survived in culture for 2 months without additional growth factors. Freshly isolated PMs were susceptible to HIV-1 R5 strains but not to X4-T-cell line-adapted strains. Interestingly, after 7 days in culture, PMs acquired susceptibility to X4-T-cell line-adapted strains. HIV entry inhibitors, TAK779 and AMD3100, blocked HIV infection of PMs, indicating that infection by R5 and X4 strains was mediated by CCR5 and CXCR4, respectively. Although PMs did not express detectable cell surface levels of CXCR4 and CCR5, they did express mRNAs of these HIV coreceptors and responded to stimulation by their natural ligands, SDF-1alpha and RANTES. PMs were susceptible to HIV-1 X4, R5, and X4R5 primary isolates. PMs after 7 days in culture produced greater amounts of X4 and X4R5 HIV than freshly isolated PMs. The day-7 PMs were more susceptible to R5 infection in a single-cycle infection assay, but there was no increase in viral production in a multiple-round infection assay. The level of CXCR4 mRNA and production of CC-chemokines (MIP-1alpha, MIP-1beta, and RANTES) increased significantly during 7 days in culture. Our results indicate that PMs are susceptible to receptor-mediated infection by a broad range of HIV strains. These primary macrophages could provide a valuable system for investigating the role of primary macrophages in HIV pathogenesis.
Figures
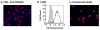

Similar articles
-
The CCR5 and CXCR4 coreceptors are both used by human immunodeficiency virus type 1 primary isolates from subtype C.J Virol. 2003 Apr;77(7):4449-56. doi: 10.1128/jvi.77.7.4449-4456.2003. J Virol. 2003. PMID: 12634405 Free PMC article.
-
Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism.J Virol. 1999 Sep;73(9):7117-25. doi: 10.1128/JVI.73.9.7117-7125.1999. J Virol. 1999. PMID: 10438797 Free PMC article.
-
HIV-1 gp120 and chemokines activate ion channels in primary macrophages through CCR5 and CXCR4 stimulation.Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4832-7. doi: 10.1073/pnas.090521697. Proc Natl Acad Sci U S A. 2000. PMID: 10758170 Free PMC article.
-
[Viral entry as therapeutic target. Current situation of entry inhibitors].Enferm Infecc Microbiol Clin. 2008 Oct;26 Suppl 11:5-11. doi: 10.1016/s0213-005x(08)76557-1. Enferm Infecc Microbiol Clin. 2008. PMID: 19133215 Review. Spanish.
-
The biology of CCR5 and CXCR4.Curr Opin HIV AIDS. 2009 Mar;4(2):96-103. doi: 10.1097/COH.0b013e328324bbec. Curr Opin HIV AIDS. 2009. PMID: 19339947 Free PMC article. Review.
Cited by
-
Brain Microglial Cells Are Highly Susceptible to HIV-1 Infection and Spread.AIDS Res Hum Retroviruses. 2017 Nov;33(11):1155-1165. doi: 10.1089/AID.2017.0004. Epub 2017 Jun 26. AIDS Res Hum Retroviruses. 2017. PMID: 28486838 Free PMC article.
-
HIV-1 induces telomerase activity in monocyte-derived macrophages, possibly safeguarding one of its reservoirs.J Virol. 2012 Oct;86(19):10327-37. doi: 10.1128/JVI.01495-12. Epub 2012 Jul 11. J Virol. 2012. PMID: 22787205 Free PMC article.
-
Functional Phenotypes of Peritoneal Macrophages Upon AMD3100 Treatment During Colitis-Associated Tumorigenesis.Front Med (Lausanne). 2022 May 9;9:840704. doi: 10.3389/fmed.2022.840704. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35615089 Free PMC article.
References
-
- Cassol E, Alfano M, Biswas P, Poli G. Monocyte-derived macrophages and myeloid cell lines as targets of HIV-1 replication and persistence. J Leukoc Biol Nov. 2006;80(5):1018–1030. - PubMed
-
- Gorry PR, Churchill M, Crowe SM, Cunningham AL, Gabuzda D. Pathogenesis of macrophage tropic HIV-1. Curr HIV Res Jan. 2005;3(1):53–60. - PubMed
-
- Kedzierska K, Crowe SM. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem Nov. 2002;9(21):1893–1903. - PubMed
-
- Clarke JR, White NC, Weber JN. HIV Compartmentalization: Pathogenesis and Clinical Implications. AIDS Rev. 2000;2:15–22.
-
- Igarashi T, Brown CR, Endo Y, et al. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):658–663. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

