Symmetry breaking during Drosophila oogenesis
- PMID: 20066085
- PMCID: PMC2742093
- DOI: 10.1101/cshperspect.a001891
Symmetry breaking during Drosophila oogenesis
Abstract
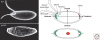
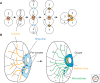
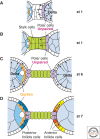
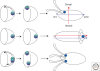
The orthogonal axes of Drosophila are established during oogenesis through a hierarchical series of symmetry-breaking steps, most of which can be traced back to asymmetries inherent in the architecture of the ovary. Oogenesis begins with the formation of a germline cyst of 16 cells connected by ring canals. Two of these 16 cells have four ring canals, whereas the others have fewer. The first symmetry-breaking step is the selection of one of these two cells to become the oocyte. Subsequently, the germline cyst becomes surrounded by somatic follicle cells to generate individual egg chambers. The second symmetry-breaking step is the posterior positioning of the oocyte within the egg chamber, a process mediated by adhesive interactions with a special group of somatic cells. Posterior oocyte positioning is accompanied by a par gene-dependent repolarization of the microtubule network, which establishes the posterior cortex of the oocyte. The next two steps of symmetry breaking occur during midoogenesis after the volume of the oocyte has increased about 10-fold. First, a signal from the oocyte specifies posterior follicle cells, polarizing a symmetric prepattern present within the follicular epithelium. Second, the posterior follicle cells send a signal back to the oocyte, which leads to a second repolarization of the oocyte microtubule network and the asymmetric migration of the oocyte nucleus. This process again requires the par genes. The repolarization of the microtubule network results in the transport of bicoid and oskar mRNAs, the anterior and posterior determinants, respectively, of the embryonic axis, to opposite poles of the oocyte. The asymmetric positioning of the oocyte nucleus defines a cortical region of the oocyte where gurken mRNA is localized, thus breaking the dorsal-ventral symmetry of the egg and embryo.
Figures
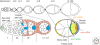
References
-
- Assa-Kunik E, Torres IL, Schejter ED, Johnston DS, Shilo BZ 2007. Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development 134:1161–1169 - PubMed
-
- Becam IE, Tanentzapf G, Lepesant JA, Brown NH, Huynh JR 2005. Integrin-independent repression of cadherin transcription by talin during axis formation in Drosophila. Nat Cell Biol 7:510–516 - PubMed
-
- Benton R, St Johnston D 2003. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115:691–704 - PubMed
-
- Benton R, Palacios IM, St Johnston D 2002. Drosophila 14-3-3/PAR-5 is an essential mediator of PAR-1 function in axis formation. Dev Cell 3:659–671 - PubMed
-
- Bolivar J, Huynh JR, Lopez-Schier H, Gonzalez C, St Johnston D, Gonzalez-Reyes A 2001. Centrosome migration into the Drosophila oocyte is independent of BicD and egl, and of the organisation of the microtubule cytoskeleton. Development 128:1889–1897 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
