Vertebrate limb development: moving from classical morphogen gradients to an integrated 4-dimensional patterning system
- PMID: 20066096
- PMCID: PMC2773624
- DOI: 10.1101/cshperspect.a001339
Vertebrate limb development: moving from classical morphogen gradients to an integrated 4-dimensional patterning system
Abstract
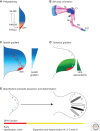
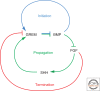
A wealth of classical embryological manipulation experiments taking mainly advantage of the chicken limb buds identified the apical ectodermal ridge (AER) and the zone of polarizing activity (ZPA) as the respective ectodermal and mesenchymal key signaling centers coordinating proximodistal (PD) and anteroposterior (AP) limb axis development. These experiments inspired Wolpert's French flag model, which is a classic among morphogen gradient models. Subsequent molecular and genetic analysis in the mouse identified retinoic acid as proximal signal, and fibroblast growth factors (FGFs) and sonic hedgehog (SHH) as the essential instructive signals produced by AER and ZPA, respectively. Recent studies provide good evidence that progenitors are specified early with respect to their PD and AP fates and that morpho-regulatory signaling is also required for subsequent proliferative expansion of the specified progenitor pools. The determination of particular fates seems to occur rather late and depends on additional signals such as bone morphogenetic proteins (BMPs), which indicates that cells integrate signaling inputs over time and space. The coordinate regulation of PD and AP axis patterning is controlled by an epithelial-mesenchymal feedback signaling system, in which transcriptional regulation of the BMP antagonist Gremlin1 integrates inputs from the BMP, SHH, and FGF pathways. Vertebrate limb-bud development is controlled by a 4-dimensional (4D) patterning system integrating positive and negative regulatory feedback loops, rather than thresholds set by morphogen gradients.
Figures



Similar articles
-
How the embryo makes a limb: determination, polarity and identity.J Anat. 2015 Oct;227(4):418-30. doi: 10.1111/joa.12361. Epub 2015 Aug 7. J Anat. 2015. PMID: 26249743 Free PMC article. Review.
-
Apical ectodermal ridge regulates three principal axes of the developing limb.J Zhejiang Univ Sci B. 2020 Oct.;21(10):757-766. doi: 10.1631/jzus.B2000285. J Zhejiang Univ Sci B. 2020. PMID: 33043642 Free PMC article. Review.
-
Limb patterning: from signaling gradients to molecular oscillations.J Mol Biol. 2014 Feb 20;426(4):780-4. doi: 10.1016/j.jmb.2013.11.022. Epub 2013 Dec 4. J Mol Biol. 2014. PMID: 24316003
-
Shh, Bmp-2 and Hoxd-13 gene expression in chick limb bud cells in culture.Dev Growth Differ. 1998 Aug;40(4):457-64. doi: 10.1046/j.1440-169x.1998.t01-2-00011.x. Dev Growth Differ. 1998. PMID: 9727360
-
Synergistic effects of FGF and non-ridge ectoderm on gene expression involved in the formation of the anteroposterior axis of the chick limb bud in cell culture.Dev Growth Differ. 2000 Jun;42(3):219-27. doi: 10.1046/j.1440-169x.2000.00512.x. Dev Growth Differ. 2000. PMID: 10910128
Cited by
-
SREBP-2-deficient and hypomorphic mice reveal roles for SREBP-2 in embryonic development and SREBP-1c expression.J Lipid Res. 2016 Mar;57(3):410-21. doi: 10.1194/jlr.M064022. Epub 2015 Dec 18. J Lipid Res. 2016. PMID: 26685326 Free PMC article.
-
Amelia: a multi-center descriptive epidemiologic study in a large dataset from the International Clearinghouse for Birth Defects Surveillance and Research, and overview of the literature.Am J Med Genet C Semin Med Genet. 2011 Nov 15;157C(4):288-304. doi: 10.1002/ajmg.c.30319. Epub 2011 Oct 14. Am J Med Genet C Semin Med Genet. 2011. PMID: 22002956 Free PMC article. Review.
-
Models for the generation and interpretation of gradients.Cold Spring Harb Perspect Biol. 2009 Oct;1(4):a001362. doi: 10.1101/cshperspect.a001362. Cold Spring Harb Perspect Biol. 2009. PMID: 20066097 Free PMC article. Review.
-
Expression analysis of limb element markers during mouse embryonic development.Dev Dyn. 2018 Nov;247(11):1217-1226. doi: 10.1002/dvdy.24671. Epub 2018 Nov 7. Dev Dyn. 2018. PMID: 30225906 Free PMC article.
-
Radiological imaging of congenital hand anomalies--a 6-year single-centre experience and what the hand surgeons want to know.Skeletal Radiol. 2015 Apr;44(4):549-56. doi: 10.1007/s00256-014-2084-2. Epub 2014 Dec 19. Skeletal Radiol. 2015. PMID: 25522683
References
-
- Ahn S, Joyner AL 2004. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118:505–516 - PubMed
-
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw III EB 2001. BMPR–IA signaling is required for the formation of the apical ectodermal ridge and dorsal–ventral patterning of the limb. Development 128:4449–4461 - PubMed
-
- Barna M, Niswander L 2007. Visualization of cartilage formation: Insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev Cell 12:931–941 - PubMed
-
- Bénazet JD, Bischofberger M, Tiecke E, Gonçalves A, Martin JF, Zuniga A, Naef F, Zeller R 2009. A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. Science 323:1050–1053 - PubMed
-
- Boulet AM, Moon AM, Arenkiel BR, Capecchi MR 2004. The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev Biol 273:361–372 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
