Listeria monocytogenes internalin and E-cadherin: from bench to bedside
- PMID: 20066101
- PMCID: PMC2773623
- DOI: 10.1101/cshperspect.a003087
Listeria monocytogenes internalin and E-cadherin: from bench to bedside
Abstract
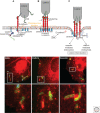
Listeria monocytogenes is a Gram-positive bacterium responsible for a severe infection associated with different clinical features (gastroenteritis, meningoencephalitis, and abortion in pregnant women). These pathologies are caused by the unusual capacity of the bacterium to cross three host barriers during infection and to invade nonphagocytic cells. To invade host cells, Listeria uses two proteins, InlA and InlB, which have specific receptors on the host-cell surface, E-cadherin and Met, respectively. Here, we discuss the specificity of the InlA-E-cadherin interaction, the signaling cascade activated on E-cadherin engagement by InlA, and the role of InlA and E-cadherin in the breaching of host barriers and the dissemination of the infection.
Figures



References
-
- Agerer F, Lux S, Michel A, Rohde M, Ohlsen K, Hauck CR 2005. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J Cell Sci 118:2189–2200 - PubMed
-
- Bakardjiev AI, Stacy BA, Portnoy DA 2005. Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal infection. J Infect Dis 191:1889–1897 - PubMed
-
- Bierne H, Sabet C, Personnic N, Cossart P 2007. Internalins: A complex family of leucine-rich repeat-containing proteins in Listeria monocytogenes. Microbes Infect 9:1156–1166 - PubMed
-
- Bonazzi M, Cossart P 2006. Bacterial entry into cells: A role for the endocytic machinery. FEBS Lett 580:2962–2967 - PubMed
-
- Bonazzi M, Lecuit M, Cossart P 2009. Listeria monocytogenes internalin and E-cadherin: From structure to pathogenesis. Cell Microbiol 11:693–702 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
