Structure and biochemistry of cadherins and catenins
- PMID: 20066110
- PMCID: PMC2773639
- DOI: 10.1101/cshperspect.a003053
Structure and biochemistry of cadherins and catenins
Abstract
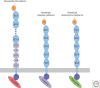
Classical cadherins mediate specific adhesion at intercellular adherens junctions. Interactions between cadherin ectodomains from apposed cells mediate cell-cell contact, whereas the intracellular region functionally links cadherins to the underlying cytoskeleton. Structural, biophysical, and biochemical studies have provided important insights into the mechanism and specificity of cell-cell adhesion by classical cadherins and their interplay with the cytoskeleton. Adhesive binding arises through exchange of beta strands between the first extracellular cadherin domains (EC1) of partner cadherins from adjacent cells. This "strand-swap" binding mode is common to classical and desmosomal cadherins, but sequence alignments suggest that other cadherins will bind differently. The intracellular region of classical cadherins binds to p120 and beta-catenin, and beta-catenin binds to the F-actin binding protein alpha-catenin. Rather than stably bridging beta-catenin to actin, it appears that alpha-catenin actively regulates the actin cytoskeleton at cadherin-based cell-cell contacts.
Figures
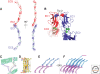
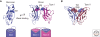



References
-
- Abe K, Chisaka O, Van Roy F, Takeichi M 2004. Stability of dendritic spines and synaptic contacts is controlled by α-N-catenin. Nat Neurosci 7:357–363 - PubMed
-
- Aberle H, Schwartz H, Hoschuetzky H, Kemler R 1996. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to α-catenin. J Biol Chem 271:1520–1526 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
