Robust generation and decoding of morphogen gradients
- PMID: 20066119
- PMCID: PMC2773648
- DOI: 10.1101/cshperspect.a001990
Robust generation and decoding of morphogen gradients
Abstract
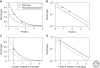
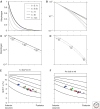
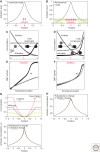
Morphogen gradients play a key role in multiple differentiation processes. Both the formation of the gradient and its interpretation by the receiving cells need to occur at high precision to ensure reproducible patterning. This need for quantitative precision is challenged by fluctuations in the environmental conditions and by variations in the genetic makeup of the developing embryos. We discuss mechanisms that buffer morphogen profiles against variations in gene dosage. Self-enhanced morphogen degradation and pre-steady-state decoding provide general means for buffering the morphogen profile against fluctuations in morphogen production rate. A more specific "shuttling" mechanism, which establishes a sharp and robust activation profile of a widely expressed morphogen, and enables the adjustment of morphogen profile with embryo size, is also described. Finally, we consider the transformation of the smooth gradient profile into sharp borders of gene expression in the signal-receiving cells. The integration theory and experiments are increasingly used, providing key insights into the system-level functioning of the developmental system.
Figures

References
-
- Barkai N, Ben-Zvi D 2009. ‘Big frog, small frog’–maintaining proportions in embryonic development: Delivered on 2 July 2008 at the 33rd FEBS Congress in Athens, Greece. FEBS J 276:1196–1207 - PubMed
-
- Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N 2008. Scaling of the BMP activation gradient in Xenopus embryos. Nature 453:1205–1211 - PubMed
-
- Bergmann S, Tamari Z, Schejter E, Shilo BZ, Barkai N 2008. Re-examining the stability of the Bicoid morphogen gradient. Cell 132:15–17; author reply 17–18 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
