Expression and function of TNF and IL-1 receptors on human regulatory T cells
- PMID: 20066156
- PMCID: PMC2799662
- DOI: 10.1371/journal.pone.0008639
Expression and function of TNF and IL-1 receptors on human regulatory T cells
Abstract
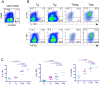
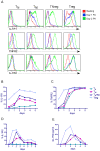
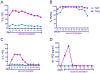
Regulatory T cells (Tregs) suppress immune activation and are critical in preventing autoimmune diseases. While the ability of Tregs to inhibit proliferation of other T cells is well established, it is not yet clear whether Tregs also modulate inflammatory cytokines during an immune response. Here, we show that the expression of inflammatory cytokine receptors IL-1R1 and TNFR2 were higher on resting mature Tregs compared to naïve or memory T cells. While upon activation through the T cell receptor (TCR), expression of IL-1R1 and TNFR2 were upregulated on all T cell subsets, IL-1R1 maintained significantly higher expression on activated Tregs as compared to other T cell subsets. The decoy receptor for IL-1 (IL-1R2) was not expressed by any of the resting T cells but was rapidly upregulated and preferentially expressed upon TCR-stimulation on Tregs. In addition, we found that Tregs also expressed high levels of mRNA for IL-1 antagonist, IL-1RA. TCR-stimulation of naïve T cells in the presence of TGFbeta, which induces FOXP3 expression, however did not result in upregulation of IL-1R1 or IL-1R2. In addition, ectopic expression of FOXP3 in non-Tregs, while causing significant upregulation of IL-1R1 and IL-1R2, did not achieve the levels seen in bona fide Tregs. We also determined that resting human Tregs expressing IL-1R1 did not have higher suppressive capacity compared to IL-1R1- Tregs, suggesting that IL-1R1 does not discriminate suppressive resting Tregs in healthy individuals. Functionally, activated human Tregs displayed a capacity to neutralize IL-1beta, which suggests a physiological significance for the expression of IL-1 decoy receptor on Tregs. In conclusion, our findings that human Tregs preferentially express receptors for TNF and IL-1 suggest a potential function in sensing and dampening local inflammation.
Conflict of interest statement
Figures




Similar articles
-
Expression of costimulatory TNFR2 induces resistance of CD4+FoxP3- conventional T cells to suppression by CD4+FoxP3+ regulatory T cells.J Immunol. 2010 Jul 1;185(1):174-82. doi: 10.4049/jimmunol.0903548. Epub 2010 Jun 4. J Immunol. 2010. PMID: 20525892 Free PMC article.
-
Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells.J Immunol. 2007 Jul 1;179(1):154-61. doi: 10.4049/jimmunol.179.1.154. J Immunol. 2007. PMID: 17579033
-
TNF optimally activatives regulatory T cells by inducing TNF receptor superfamily members TNFR2, 4-1BB and OX40.Eur J Immunol. 2011 Jul;41(7):2010-20. doi: 10.1002/eji.201041205. Eur J Immunol. 2011. PMID: 21491419 Free PMC article.
-
The Significance of Tumor Necrosis Factor Receptor Type II in CD8+ Regulatory T Cells and CD8+ Effector T Cells.Front Immunol. 2018 Mar 22;9:583. doi: 10.3389/fimmu.2018.00583. eCollection 2018. Front Immunol. 2018. PMID: 29623079 Free PMC article. Review.
-
TNF-alpha: an activator of CD4+FoxP3+TNFR2+ regulatory T cells.Curr Dir Autoimmun. 2010;11:119-34. doi: 10.1159/000289201. Epub 2010 Feb 18. Curr Dir Autoimmun. 2010. PMID: 20173391 Free PMC article. Review.
Cited by
-
Mobilized Multipotent Hematopoietic Progenitors Promote Expansion and Survival of Allogeneic Tregs and Protect Against Graft Versus Host Disease.Front Immunol. 2021 Feb 12;11:607180. doi: 10.3389/fimmu.2020.607180. eCollection 2020. Front Immunol. 2021. PMID: 33643294 Free PMC article.
-
Association between IL-1R2 polymorphisms and lung cancer risk in the Chinese Han population: A case-control study.Mol Genet Genomic Med. 2019 May;7(5):e644. doi: 10.1002/mgg3.644. Epub 2019 Mar 20. Mol Genet Genomic Med. 2019. PMID: 30895747 Free PMC article.
-
Immune suppressive landscape in the human esophageal squamous cell carcinoma microenvironment.Nat Commun. 2020 Dec 8;11(1):6268. doi: 10.1038/s41467-020-20019-0. Nat Commun. 2020. PMID: 33293583 Free PMC article.
-
TNFR2 Expression on CD25(hi)FOXP3(+) T Cells Induced upon TCR Stimulation of CD4 T Cells Identifies Maximal Cytokine-Producing Effectors.Front Immunol. 2013 Aug 6;4:233. doi: 10.3389/fimmu.2013.00233. eCollection 2013. Front Immunol. 2013. PMID: 23964278 Free PMC article.
-
The effect of cellular isolation and cryopreservation on the expression of markers identifying subsets of regulatory T cells.J Immunol Methods. 2016 Apr;431:31-7. doi: 10.1016/j.jim.2016.02.004. Epub 2016 Feb 19. J Immunol Methods. 2016. PMID: 26855370 Free PMC article.
References
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. - PubMed
-
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. - PubMed
-
- Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–98; discussion 98–105, 106–114. - PubMed
-
- Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, et al. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–5133. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

