PPARbeta activation inhibits melanoma cell proliferation involving repression of the Wilms' tumour suppressor WT1
- PMID: 20066433
- PMCID: PMC2842567
- DOI: 10.1007/s00424-009-0776-6
PPARbeta activation inhibits melanoma cell proliferation involving repression of the Wilms' tumour suppressor WT1
Abstract
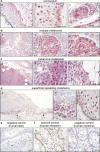
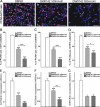
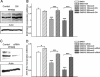
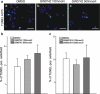
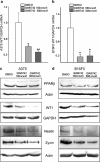
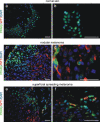
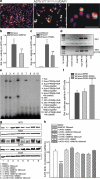
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that strongly influence molecular signalling in normal and cancer cells. Although increasing evidence suggests a role of PPARs in skin carcinogenesis, only expression of PPARgamma has been investigated in human melanoma tissues. Activation of PPARalpha has been shown to inhibit the metastatic potential, whereas stimulation of PPARgamma decreased melanoma cell proliferation. We show here that the third member of the PPAR family, PPARbeta/delta is expressed in human melanoma samples. Specific pharmacological activation of PPARbeta using GW0742 or GW501516 in low concentrations inhibits proliferation of human and murine melanoma cells. Inhibition of proliferation is accompanied by decreased expression of the Wilms' tumour suppressor 1 (WT1), which is implicated in melanoma proliferation. We demonstrate that PPARbeta directly represses WT1 as (1) PPARbeta activation represses WT1 promoter activity; (2) in chromatin immunoprecipitation and electrophoretic mobility shift assays, we identified a binding element for PPARbeta in the WT1 promoter; (3) deletion of this binding element abolishes repression by PPARbeta and (4) the WT1 downstream molecules nestin and zyxin are down-regulated upon PPARbeta activation. Our findings elucidate a novel mechanism of signalling by ligands of PPARbeta, which leads to suppression of melanoma cell growth through direct repression of WT1.
Figures
Similar articles
-
Expression of the Wilms' tumour gene and its association with PPARβ/δ in healthy skin and melanoma of horses.Acta Vet Hung. 2021 Jan 15;68(4):374-379. doi: 10.1556/004.2020.00045. Acta Vet Hung. 2021. PMID: 33459615
-
The Wilms' tumor suppressor WT1 is associated with melanoma proliferation.Pflugers Arch. 2008 Feb;455(5):839-47. doi: 10.1007/s00424-007-0340-1. Epub 2007 Oct 3. Pflugers Arch. 2008. PMID: 17912546
-
Peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) ligands inhibit growth of UACC903 and MCF7 human cancer cell lines.Toxicology. 2008 Jan 14;243(1-2):236-43. doi: 10.1016/j.tox.2007.10.023. Epub 2007 Nov 4. Toxicology. 2008. PMID: 18054822 Free PMC article.
-
Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) acts as regulator of metabolism linked to multiple cellular functions.Pharmacol Ther. 2010 Mar;125(3):423-35. doi: 10.1016/j.pharmthera.2009.12.001. Epub 2009 Dec 22. Pharmacol Ther. 2010. PMID: 20026355 Review.
-
Regulatory mechanisms mediated by peroxisome proliferator-activated receptor-β/δ in skin cancer.Mol Carcinog. 2019 Sep;58(9):1612-1622. doi: 10.1002/mc.23033. Epub 2019 May 6. Mol Carcinog. 2019. PMID: 31062422 Free PMC article. Review.
Cited by
-
Fatty Acids of CLA-Enriched Egg Yolks Can Induce Transcriptional Activation of Peroxisome Proliferator-Activated Receptors in MCF-7 Breast Cancer Cells.PPAR Res. 2017;2017:2865283. doi: 10.1155/2017/2865283. Epub 2017 Mar 26. PPAR Res. 2017. PMID: 28458685 Free PMC article.
-
PPARdelta in Affected Atopic Dermatitis and Psoriasis: A Possible Role in Metabolic Reprograming.Int J Mol Sci. 2021 Jul 8;22(14):7354. doi: 10.3390/ijms22147354. Int J Mol Sci. 2021. PMID: 34298981 Free PMC article. Review.
-
PPARs and the Kynurenine Pathway in Melanoma-Potential Biological Interactions.Int J Mol Sci. 2023 Feb 4;24(4):3114. doi: 10.3390/ijms24043114. Int J Mol Sci. 2023. PMID: 36834531 Free PMC article. Review.
-
Ligand-activated PPARδ modulates the migration and invasion of melanoma cells by regulating Snail expression.Am J Cancer Res. 2014 Nov 19;4(6):674-82. eCollection 2014. Am J Cancer Res. 2014. PMID: 25520859 Free PMC article.
-
Transcriptional and functional regulation of cell cycle and UV response by PPARβ in human skin epidermal cells.FASEB J. 2024 Dec 15;38(23):e70212. doi: 10.1096/fj.202401950R. FASEB J. 2024. PMID: 39620966 Free PMC article.
References
-
- Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

