Inositol trisphosphate 3-kinases: focus on immune and neuronal signaling
- PMID: 20066467
- PMCID: PMC11115942
- DOI: 10.1007/s00018-009-0238-5
Inositol trisphosphate 3-kinases: focus on immune and neuronal signaling
Abstract
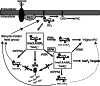
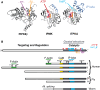
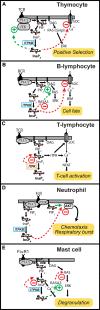
The localized control of second messenger levels sculpts dynamic and persistent changes in cell physiology and structure. Inositol trisphosphate [Ins(1,4,5)P(3)] 3-kinases (ITPKs) phosphorylate the intracellular second messenger Ins(1,4,5)P(3). These enzymes terminate the signal to release Ca(2+) from the endoplasmic reticulum and produce the messenger inositol tetrakisphosphate [Ins(1,3,4,5)P(4)]. Independent of their enzymatic activity, ITPKs regulate the microstructure of the actin cytoskeleton. The immune phenotypes of ITPK knockout mice raise new questions about how ITPKs control inositol phosphate lifetimes within spatial and temporal domains during lymphocyte maturation. The intense concentration of ITPK on actin inside the dendritic spines of pyramidal neurons suggests a role in signal integration and structural plasticity in the dendrite, and mice lacking neuronal ITPK exhibit memory deficits. Thus, the molecular and anatomical features of ITPKs allow them to regulate the spatiotemporal properties of intracellular signals, leading to the formation of persistent molecular memories.
Figures
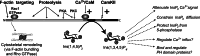
Similar articles
-
Inositol-1,4,5-trisphosphate 3-kinase A regulates dendritic morphology and shapes synaptic Ca2+ transients.Cell Signal. 2012 Mar;24(3):750-7. doi: 10.1016/j.cellsig.2011.11.010. Epub 2011 Nov 18. Cell Signal. 2012. PMID: 22120525
-
Structural and catalytic analyses of the InsP6 kinase activities of higher plant ITPKs.FASEB J. 2022 Jul;36(7):e22380. doi: 10.1096/fj.202200393R. FASEB J. 2022. PMID: 35635723 Free PMC article.
-
Inositol(1,4,5)P3 3-kinase isoenzymes: Catalytic properties and importance of targeting to F-actin to understand function.Adv Biol Regul. 2016 Jan;60:135-143. doi: 10.1016/j.jbior.2015.09.004. Epub 2015 Sep 26. Adv Biol Regul. 2016. PMID: 26446452 Review.
-
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol trisphosphate phosphorylation and shuttles actively between nucleus and cytoplasm.J Biol Chem. 2003 May 30;278(22):19765-76. doi: 10.1074/jbc.M211059200. Epub 2003 Mar 20. J Biol Chem. 2003. PMID: 12649294
-
Ins(1,4,5)P3 metabolism and the family of IP3-3Kinases.Cell Signal. 2004 Jun;16(6):643-54. doi: 10.1016/j.cellsig.2003.10.009. Cell Signal. 2004. PMID: 15093605 Review.
Cited by
-
The Concise Guide to PHARMACOLOGY 2013/14: enzymes.Br J Pharmacol. 2013 Dec;170(8):1797-867. doi: 10.1111/bph.12451. Br J Pharmacol. 2013. PMID: 24528243 Free PMC article.
-
Inositol phosphate kinases: Expanding the biological significance of the universal core of the protein kinase fold.Adv Biol Regul. 2019 Jan;71:118-127. doi: 10.1016/j.jbior.2018.10.006. Epub 2018 Oct 27. Adv Biol Regul. 2019. PMID: 30392847 Free PMC article. Review.
-
Design and Synthesis of an Inositol Phosphate Analog Based on Computational Docking Studies.Tetrahedron. 2014 Jan 28;70(4):984-990. doi: 10.1016/j.tet.2013.11.092. Tetrahedron. 2014. PMID: 25110363 Free PMC article.
-
IP3 3-kinase opposes NGF driven neurite outgrowth.PLoS One. 2012;7(2):e32386. doi: 10.1371/journal.pone.0032386. Epub 2012 Feb 22. PLoS One. 2012. PMID: 22384237 Free PMC article.
-
IP3 3-kinase B controls hematopoietic stem cell homeostasis and prevents lethal hematopoietic failure in mice.Blood. 2015 Apr 30;125(18):2786-97. doi: 10.1182/blood-2014-06-583187. Epub 2015 Mar 18. Blood. 2015. PMID: 25788703 Free PMC article.
References
-
- Kummerow C, Junker C, Kruse K, Rieger H, Quintana A, Hoth M. The immunological synapse controls local and global calcium signals in T lymphocytes. Immunol Rev. 2009;231:132–147. - PubMed
-
- Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat Rev Neurosci. 2005;6:423–434. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

