Anti-HER2 immunoliposomes for selective delivery of electron paramagnetic resonance imaging probes to HER2-overexpressing breast tumor cells
- PMID: 20066490
- PMCID: PMC4041539
- DOI: 10.1007/s10549-009-0715-4
Anti-HER2 immunoliposomes for selective delivery of electron paramagnetic resonance imaging probes to HER2-overexpressing breast tumor cells
Abstract
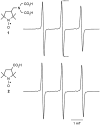
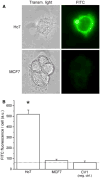
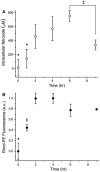
Electron paramagnetic resonance (EPR) imaging is an emerging modality that can detect and localize paramagnetic molecular probes (so-called spin probes) in vivo. We previously demonstrated that nitroxide spin probes can be encapsulated in liposomes at concentrations exceeding 100 mM, at which nitroxides exhibit a concentration-dependent quenching of their EPR signal that is analogous to the self-quenching of fluorescent molecules. Therefore, intact liposomes encapsulating high concentrations of nitroxides exhibit greatly attenuated EPR spectral signals, and endocytosis of such liposomes represents a cell-activated contrast-generating mechanism. After endocytosis, the encapsulated nitroxide is liberated and becomes greatly diluted in the intracellular milieu. This dequenches the nitroxides to generate a robust intracellular EPR signal. It is therefore possible to deliver a high concentration of nitroxides to cells while minimizing background signal from unendocytosed liposomes. We report here that intracellular EPR signal can be selectively generated in a specific cell type by exploiting its expression of Human Epidermal Growth Factor Receptor 2 (HER2). When targeted by anti-HER2 immunoliposomes encapsulating quenched nitroxides, Hc7 cells, which are novel HER2-overexpressing cells derived from the MCF7 breast tumor cell line, endocytose the liposomes copiously, in contrast to the parent MCF7 cells or control CV1 cells, which do not express HER2. HER2-dependent liposomal delivery enables Hc7 cells to accumulate 750 μM nitroxide intracellularly. Through the use of phantom models, we verify that this concentration of nitroxides is more than sufficient for EPR imaging, thus laying the foundation for using EPR imaging to visualize HER2-overexpressing Hc7 tumors in animals.
Figures




References
-
- Halpern HJ, Spencer DP, Vanpolen J, et al. Imaging radio-frequency electron-spin-resonance spectrometer with high-resolution and sensitivity for in vivo measurements. Rev Sci Instrum. 1989;60:1040–1050.
-
- Carrington A, McLachlan A. Introduction to magnetic resonance. Harper & Row; New York: 1967. pp. 1–4.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

