Therapeutic HPV DNA vaccines
- PMID: 20066511
- PMCID: PMC2891127
- DOI: 10.1007/s12026-009-8141-6
Therapeutic HPV DNA vaccines
Abstract
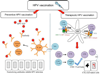
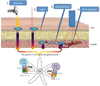
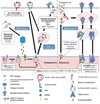
It is now well established that most cervical cancers are causally associated with HPV infection. This realization has led to efforts to control HPV-associated malignancy through prevention or treatment of HPV infection. Currently, commercially available HPV vaccines are not designed to control established HPV infection and associated premalignant and malignant lesions. To treat and eradicate pre-existing HPV infections and associated lesions which remain prevalent in the U.S. and worldwide, effective therapeutic HPV vaccines are needed. DNA vaccination has emerged as a particularly promising form of therapeutic HPV vaccines due to its safety, stability and ability to induce antigen-specific immunity. This review focuses on improving the potency of therapeutic HPV vaccines through modification of dendritic cells (DCs) by [1] increasing the number of antigen-expressing/antigen-loaded DCs, [2] improving HPV antigen expression, processing and presentation in DCs, and [3] enhancing DC and T cell interaction. Continued improvement in therapeutic HPV DNA vaccines may ultimately lead to an effective DNA vaccine for the treatment of HPV-associated malignancies.
Figures
Similar articles
-
Therapeutic DNA Vaccines for Human Papillomavirus and Associated Diseases.Hum Gene Ther. 2018 Sep;29(9):971-996. doi: 10.1089/hum.2017.197. Epub 2018 Mar 16. Hum Gene Ther. 2018. PMID: 29316817 Free PMC article.
-
Therapeutic HPV DNA vaccines.Expert Rev Vaccines. 2009 Sep;8(9):1221-35. doi: 10.1586/erv.09.76. Expert Rev Vaccines. 2009. PMID: 19722895 Free PMC article. Review.
-
DNA vaccines for cervical cancer: from bench to bedside.Exp Mol Med. 2007 Dec 31;39(6):679-89. doi: 10.1038/emm.2007.74. Exp Mol Med. 2007. PMID: 18160838 Free PMC article. Review.
-
Therapeutic vaccination for HPV induced cervical cancers.Dis Markers. 2007;23(4):337-52. doi: 10.1155/2007/245146. Dis Markers. 2007. PMID: 17627067 Free PMC article. Review.
-
Therapeutic HPV vaccines.Best Pract Res Clin Obstet Gynaecol. 2018 Feb;47:59-72. doi: 10.1016/j.bpobgyn.2017.09.008. Epub 2017 Sep 28. Best Pract Res Clin Obstet Gynaecol. 2018. PMID: 29108943 Review.
Cited by
-
Cervical cancer screening, treatment and prophylaxis in Brazil: Current and future perspectives for cervical cancer elimination.Front Med (Lausanne). 2022 Aug 24;9:945621. doi: 10.3389/fmed.2022.945621. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36091685 Free PMC article. Review.
-
Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research.J Gynecol Oncol. 2016 Sep;27(5):e51. doi: 10.3802/jgo.2016.27.e51. Epub 2016 May 31. J Gynecol Oncol. 2016. PMID: 27329199 Free PMC article. Review.
-
Immunotherapy in cervical cancer: an innovative approach for better treatment outcomes.Explor Target Antitumor Ther. 2025 Mar 2;6:1002296. doi: 10.37349/etat.2025.1002296. eCollection 2025. Explor Target Antitumor Ther. 2025. PMID: 40061136 Free PMC article. Review.
-
Construction and immunological characterization of CD40L or GM-CSF incorporated Hantaan virus like particle.Oncotarget. 2016 Sep 27;7(39):63488-63503. doi: 10.18632/oncotarget.11329. Oncotarget. 2016. PMID: 27542281 Free PMC article.
-
Induction of robust cellular immunity against HPV6 and HPV11 in mice by DNA vaccine encoding for E6/E7 antigen.Hum Vaccin Immunother. 2012 Apr;8(4):470-8. doi: 10.4161/hv.19180. Epub 2012 Feb 16. Hum Vaccin Immunother. 2012. PMID: 22336879 Free PMC article.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. - PubMed
-
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. - PubMed
-
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. - PubMed
-
- Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2:59–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

