Cellular context-dependent "colors" of transforming growth factor-beta signaling
- PMID: 20067465
- PMCID: PMC11158101
- DOI: 10.1111/j.1349-7006.2009.01441.x
Cellular context-dependent "colors" of transforming growth factor-beta signaling
Abstract
Transforming growth factor (TGF)-beta signaling has interesting characteristics in the context of cancer. Although perturbations of TGF-beta signaling are strongly implicated in cancer progression, TGF-beta signaling has both tumor-suppressive and tumor-promoting effects. For example, TGF-beta inhibits cancer cell proliferation in some cellular contexts, but promotes it in others. Although several approaches to treating cancer have been considered using TGF-beta-based therapeutic strategies, the contradictory behaviors of TGF-beta have made these approaches complex. To put them to practical use, either the tumor-suppressive or tumor-promoting arm needs to be specifically manipulated. However, there is virtually no method to specifically regulate a certain cell response induced by TGF-beta. In this review, we first consider the basic machinery of TGF-beta signaling, and describe several cell responses induced by TGF-beta stimulation in specific contexts. Mechanisms by which TGF-beta can induce several responses in a cellular context-dependent fashion are discussed with established paradigms and models. We also address perspectives on the specific control of only a subset of numerous cell responses induced by TGF-beta stimulation. Such methods will aid specific regulation of either the tumor-suppressive or tumor-promoting arm of the TGF-beta pathway and in realization of TGF-beta-based treatment of malignant tumors.
Figures
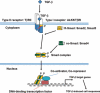

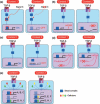
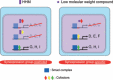
Similar articles
-
Biology of transforming growth factor-β signaling.Curr Pharm Biotechnol. 2011 Dec;12(12):2099-107. doi: 10.2174/138920111798808419. Curr Pharm Biotechnol. 2011. PMID: 21619537 Review.
-
The role for transforming growth factor-beta (TGF-beta) in human cancer.Crit Rev Oncog. 1999;10(4):303-60. Crit Rev Oncog. 1999. PMID: 10654929 Review.
-
Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation.J Invest Dermatol. 2002 Feb;118(2):211-5. doi: 10.1046/j.1523-1747.2002.01641.x. J Invest Dermatol. 2002. PMID: 11841535 Review.
-
TGF-β signal transduction spreading to a wider field: a broad variety of mechanisms for context-dependent effects of TGF-β.Cell Tissue Res. 2012 Jan;347(1):37-49. doi: 10.1007/s00441-011-1179-5. Epub 2011 May 27. Cell Tissue Res. 2012. PMID: 21618142 Review.
-
Duel nature of TGF-beta signaling: tumor suppressor vs. tumor promoter.Curr Opin Oncol. 2005 Jan;17(1):49-54. doi: 10.1097/01.cco.0000143682.45316.ae. Curr Opin Oncol. 2005. PMID: 15608513 Review.
Cited by
-
Stressin' Sestrins take an aging fight.EMBO Mol Med. 2010 Oct;2(10):388-400. doi: 10.1002/emmm.201000097. EMBO Mol Med. 2010. PMID: 20878915 Free PMC article. Review.
-
Smad2 and Smad6 as predictors of overall survival in oral squamous cell carcinoma patients.Mol Cancer. 2010 May 12;9:106. doi: 10.1186/1476-4598-9-106. Mol Cancer. 2010. PMID: 20462450 Free PMC article.
-
Truncated forms of RUNX3 Unlike Full Length Protein Alter Cell Proliferation in a TGF-β Context Dependent Manner.Iran J Pharm Res. 2017 Summer;16(3):1194-1203. Iran J Pharm Res. 2017. PMID: 29201108 Free PMC article.
-
Wound healing and cancer stem cells: inflammation as a driver of treatment resistance in breast cancer.Cancer Growth Metastasis. 2015 Jan 29;8:1-13. doi: 10.4137/CGM.S11286. eCollection 2015. Cancer Growth Metastasis. 2015. PMID: 25674014 Free PMC article. Review.
-
βig-h3 potentiates the profibrogenic effect of TGFβ signaling on connective tissue progenitor cells through the negative regulation of master chondrogenic genes.Tissue Eng Part A. 2013 Feb;19(3-4):448-57. doi: 10.1089/ten.TEA.2012.0188. Epub 2012 Oct 24. Tissue Eng Part A. 2013. PMID: 22924741 Free PMC article.
References
-
- Levy K, Hill CS. Alteration in components of the TGF‐β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 2006; 17: 41–58. - PubMed
-
- Markowitz S, Wang J, Myeroff L et al. Inactivation of the type II TGF‐β receptor in colon cancer cells with microsatellite instability. Science 1995; 268: 1336–8. - PubMed
-
- Hahn SA, Seymour AB, Hoque AT et al. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res 1995; 55: 4670–5. - PubMed
-
- Tsushima H, Kawata S, Tamura S et al. High levels of transforming growth factor β1 in patients with colorectal cancer: association with disease progression. Gastroenterology 1996; 110: 375–82. - PubMed
-
- Wikström P, Stattin P, Franck‐Lissbrant I, Damber JE, Bergh A. Transforming growth factor β1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 1998; 37: 19–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

