The mixed-lineage kinase 1-3 signalling pathway regulates stress response in cardiac myocytes via GATA-4 and AP-1 transcription factors
- PMID: 20067472
- PMCID: PMC2828035
- DOI: 10.1111/j.1476-5381.2009.00567.x
The mixed-lineage kinase 1-3 signalling pathway regulates stress response in cardiac myocytes via GATA-4 and AP-1 transcription factors
Abstract
Background and purpose: The mixed-lineage kinases (MLKs) act upstream of mitogen-activated protein kinases, but their role in cardiac biology and pathology is largely unknown.
Experimental approach: We investigated the effect of a MLK1-3 inhibitor CEP-11004 on G protein-coupled receptor agonist-induced stress response in neonatal rat cardiac myocytes in culture.
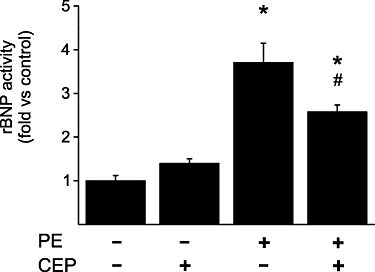
Key results: CEP-11004 administration dose-dependently attenuated phenylephrine and endothelin-1 (ET-1)-induced c-Jun N-terminal kinase activation. MLK inhibition also reduced ET-1- and phenylephrine-induced phosphorylation of p38 mitogen-activated protein kinase. In contrast, phenylephrine-induced extracellular signal-regulated kinase phosphorylation was further up-regulated by CEP-11004. ET-1 increased activator protein-1 binding activity 3.5-fold and GATA-binding protein 4 (GATA-4) binding activity 1.8-fold, both of which were attenuated with CEP-11004 administration by 59% and 63% respectively. Phenylephrine induced activator protein-1 binding activity by 2.6-fold, which was decreased by 81% with CEP-11004 administration. Phenylephrine also induced a 3.7-fold increase in the transcriptional activity of B-type natriuretic peptide (BNP), which was attenuated by 41% with CEP-11004 administration. In agreement, MLK inhibition also reduced hypertrophic agonist-induced secretion of immunoreactive atrial natriuretic peptide and BNP.
Conclusions and implications: These results showed that inhibition of the MLK1-3 signalling pathway was sufficient for suppressing the activity of key nuclear effectors (GATA-4 and activator protein-1 transcription factors) in cardiac hypertrophy, and attenuated the agonist-induced atrial natriuretic peptide secretion and activation of BNP gene transcription.
Figures




Similar articles
-
Distinct roles of mitogen-activated protein kinase pathways in GATA-4 transcription factor-mediated regulation of B-type natriuretic peptide gene.J Biol Chem. 2002 Apr 19;277(16):13752-60. doi: 10.1074/jbc.M105736200. Epub 2002 Feb 4. J Biol Chem. 2002. PMID: 11827958
-
Endothelin-1-induced cardiac hypertrophy is inhibited by activation of peroxisome proliferator-activated receptor-alpha partly via blockade of c-Jun NH2-terminal kinase pathway.Circulation. 2004 Feb 24;109(7):904-10. doi: 10.1161/01.CIR.0000112596.06954.00. Epub 2004 Feb 16. Circulation. 2004. PMID: 14967736
-
c-Jun N-terminal kinase-interacting protein 1 inhibits gene expression in response to hypertrophic agonists in neonatal rat ventricular myocytes.Biochem J. 2001 Sep 1;358(Pt 2):489-95. doi: 10.1042/0264-6021:3580489. Biochem J. 2001. PMID: 11513749 Free PMC article.
-
Activation of protein kinase cascades in the heart by hypertrophic G protein-coupled receptor agonists.Am J Cardiol. 1999 Jun 17;83(12A):64H-69H. doi: 10.1016/s0002-9149(99)00261-1. Am J Cardiol. 1999. PMID: 10750590 Review.
-
Mixed Lineage Kinase-c-Jun N-Terminal Kinase Axis: A Potential Therapeutic Target in Cancer.Genes Cancer. 2013 Sep;4(9-10):334-41. doi: 10.1177/1947601913485415. Genes Cancer. 2013. PMID: 24349631 Free PMC article. Review.
Cited by
-
Protective role of downregulated MLK3 in myocardial adaptation to chronic hypoxia.J Physiol Biochem. 2016 Aug;73(3):371-380. doi: 10.1007/s13105-017-0561-5. Epub 2017 May 30. J Physiol Biochem. 2016. PMID: 28555332
-
Systems biology and biomechanical model of heart failure.Curr Cardiol Rev. 2012 Aug;8(3):220-30. doi: 10.2174/157340312803217238. Curr Cardiol Rev. 2012. PMID: 22935019 Free PMC article. Review.
-
Mixed lineage kinase-3 prevents cardiac dysfunction and structural remodeling with pressure overload.Am J Physiol Heart Circ Physiol. 2019 Jan 1;316(1):H145-H159. doi: 10.1152/ajpheart.00029.2018. Epub 2018 Oct 26. Am J Physiol Heart Circ Physiol. 2019. PMID: 30362822 Free PMC article.
-
Molecular discoveries and treatment strategies by direct reprogramming in cardiac regeneration.Transl Res. 2019 Jan;203:73-87. doi: 10.1016/j.trsl.2018.07.012. Epub 2018 Jul 31. Transl Res. 2019. PMID: 30142308 Free PMC article. Review.
-
LncRNA XIST promotes myocardial infarction by regulating FOS through targeting miR-101a-3p.Aging (Albany NY). 2020 Apr 21;12(8):7232-7247. doi: 10.18632/aging.103072. Epub 2020 Apr 21. Aging (Albany NY). 2020. PMID: 32315985 Free PMC article.
References
-
- Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. - PubMed
-
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. - PubMed
-
- Bloem LJ, Pickard TR, Acton S, Donoghue M, Beavis RC, Knierman MD, et al. Tissue distribution and functional expression of a cDNA encoding a novel mixed lineage kinase. J Mol Cell Cardiol. 2001;33:1739–1750. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

