Gangliosides induce autophagic cell death in astrocytes
- PMID: 20067473
- PMCID: PMC2828023
- DOI: 10.1111/j.1476-5381.2009.00563.x
Gangliosides induce autophagic cell death in astrocytes
Abstract
Background and purpose: Gangliosides, sialic acid-containing glycosphingolipids, abundant in brain, are involved in neuronal function and disease, but the precise molecular mechanisms underlying their physiological or pathological activities are poorly understood. In this study, the pathological role of gangliosides in the extracellular milieu with respect to glial cell death and lipid raft/membrane disruption was investigated.
Experimental approach: We determined the effect of gangliosides on astrocyte death or survival using primary astrocyte cultures and astrocytoma/glioma cell lines as a model. Signalling pathways of ganglioside-induced autophagic cell death of astrocytes were examined using pharmacological inhibitors and biochemical and genetic assays.
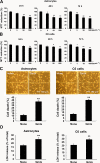
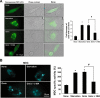
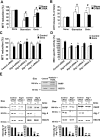
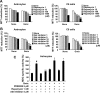
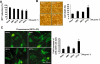
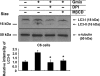
Key results: Gangliosides induced autophagic cell death in based on the following observations. Incubation of the cells with a mixture of gangliosides increased a punctate distribution of fluorescently labelled microtubule-associated protein 1 light chain 3 (GFP-LC3), the ratio of LC3-II/LC3-I and LC3 flux. Gangliosides also increased the formation of autophagic vacuoles as revealed by monodansylcadaverine staining. Ganglioside-induced cell death was inhibited by either a knockdown of beclin-1/Atg-6 or Atg-7 gene expression or by 3-methyladenine, an inhibitor of autophagy. Reactive oxygen species (ROS) were involved in ganglioside-induced autophagic cell death of astrocytes, because gangliosides induced ROS production and ROS scavengers decreased autophagic cell death. In addition, lipid rafts played an important role in ganglioside-induced astrocyte death.
Conclusions and implications: Gangliosides released under pathological conditions may induce autophagic cell death of astrocytes, identifying a neuropathological role for gangliosides.
Figures





Similar articles
-
NF-κB as a common signaling pathway in ganglioside-induced autophagic cell death and activation of astrocytes.J Neuroimmunol. 2010 Sep 14;226(1-2):66-72. doi: 10.1016/j.jneuroim.2010.05.037. Epub 2010 Jun 15. J Neuroimmunol. 2010. PMID: 20554329
-
Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia.Autophagy. 2010 Aug;6(6):738-53. doi: 10.4161/auto.6.6.12573. Epub 2010 Aug 3. Autophagy. 2010. PMID: 20574158
-
Parkinsonian toxin-induced oxidative stress inhibits basal autophagy in astrocytes via NQO2/quinone oxidoreductase 2: Implications for neuroprotection.Autophagy. 2015;11(7):1063-80. doi: 10.1080/15548627.2015.1058683. Autophagy. 2015. PMID: 26046590 Free PMC article.
-
Lysosomal acidification impairment in astrocyte-mediated neuroinflammation.J Neuroinflammation. 2025 Mar 10;22(1):72. doi: 10.1186/s12974-025-03410-w. J Neuroinflammation. 2025. PMID: 40065324 Free PMC article. Review.
-
Role of Gangliosides in Peripheral Pain Mechanisms.Int J Mol Sci. 2020 Feb 3;21(3):1005. doi: 10.3390/ijms21031005. Int J Mol Sci. 2020. PMID: 32028715 Free PMC article. Review.
Cited by
-
Sphingolipids: regulators of crosstalk between apoptosis and autophagy.J Lipid Res. 2013 Jan;54(1):5-19. doi: 10.1194/jlr.R031278. Epub 2012 Nov 13. J Lipid Res. 2013. PMID: 23152582 Free PMC article. Review.
-
Pregnenolone sulfate and cortisol induce secretion of acyl-CoA-binding protein and its conversion into endozepines from astrocytes.J Biol Chem. 2010 Jul 9;285(28):21359-65. doi: 10.1074/jbc.M110.105858. Epub 2010 May 7. J Biol Chem. 2010. PMID: 20452969 Free PMC article.
-
Glycosphingolipid metabolism and its role in ageing and Parkinson's disease.Glycoconj J. 2022 Feb;39(1):39-53. doi: 10.1007/s10719-021-10023-x. Epub 2021 Nov 10. Glycoconj J. 2022. PMID: 34757540 Free PMC article. Review.
-
Gintonin stimulates autophagic flux in primary cortical astrocytes.J Ginseng Res. 2020 Jan;44(1):67-78. doi: 10.1016/j.jgr.2018.08.004. Epub 2018 Aug 27. J Ginseng Res. 2020. PMID: 32148391 Free PMC article.
-
SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling.Biochim Biophys Acta Mol Basis Dis. 2021 Dec 1;1867(12):166260. doi: 10.1016/j.bbadis.2021.166260. Epub 2021 Aug 27. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 34461258 Free PMC article.
References
-
- Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, et al. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–35246. - PubMed
-
- Avrova NF, Victorov IV, Tyurin VA, Zakharova IO, Sokolova TV, Andreeva NA, et al. Inhibition of glutamate-induced intensification of free radical reactions by gangliosides: possible role in their protective effect in rat cerebellar granule cells and brain synaptosomes. Neurochem Res. 1998;23:945–952. - PubMed
-
- Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–510. - PubMed
-
- Barres BA, Barde Y. Neuronal and glial cell biology. Curr Opin Neurobiol. 2000;10:642–648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

