The ecto-nucleotidase NTPDase1 differentially regulates P2Y1 and P2Y2 receptor-dependent vasorelaxation
- PMID: 20067476
- PMCID: PMC2828022
- DOI: 10.1111/j.1476-5381.2009.00566.x
The ecto-nucleotidase NTPDase1 differentially regulates P2Y1 and P2Y2 receptor-dependent vasorelaxation
Abstract
Background and purpose: Extracellular nucleotides produce vasodilatation through endothelial P2 receptor activation. As these autacoids are actively metabolized by the ecto-nucleotidase nucleoside triphosphate diphosphohydrolase-1 (NTPDase1), we studied the effects of this cell surface enzyme on nucleotide-dependent vasodilatation.
Experimental approach: Vascular NTPDase expression and activity were evaluated by immunohistochemistry and histochemistry. The vascular effects of nucleotides were tested in vivo by monitoring mean arterial pressure, and in vitro comparing reactivity of aortic rings using wild-type and Entpd1(-/-) (lacking NTPDase1) mice.
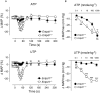
Key results: The absence of NTPDase1 in Entpd1(-/-) mice led to a dramatic drop in endothelial nucleotidase activity. This deficit was associated with an exacerbated decrease in blood pressure after nucleotide injection. Following ATP injection, mean arterial pressure was decreased in Entpd1(+/+) and Entpd1(-/-) mice by 5.0 and 17%, respectively, and by 0.1 and 19% after UTP injection (10 nmole.kg(-1) both). In vitro, the concentration-response curves of relaxation to ADP and ATP were shifted to the left, revealing a facilitation of endothelial P2Y1 and P2Y2 receptor activation in Entpd1(-/-) mice. EC(50) values in Entpd1(+/+) versus Entpd1(-/-) aortic rings were 14 microM versus 0.35 microM for ADP, and 29 microM versus 1 microM for ATP. In Entpd1(-/-) aortas, P2Y1 receptors were more extensively desensitized than P2Y2 receptors. Relaxations to the non-hydrolysable analogues ADPbetaS (P2Y1) and ATPgammaS (P2Y2) were equivalent in both genotypes confirming the normal functionality of these P2Y receptors in mutant mice.
Conclusions and implications: NTPDase1 controls endothelial P2Y receptor-dependent relaxation, regulating both agonist level and P2 receptor reactivity.
Figures



Similar articles
-
Endothelium-dependent relaxation evoked by ATP and UTP in the aorta of P2Y2-deficient mice.Br J Pharmacol. 2006 Mar;147(5):569-74. doi: 10.1038/sj.bjp.0706642. Br J Pharmacol. 2006. PMID: 16415908 Free PMC article.
-
Comparison of P2 receptor subtypes producing dilation in rat intracerebral arterioles.Stroke. 2003 Jun;34(6):1473-8. doi: 10.1161/01.STR.0000071527.10129.65. Epub 2003 May 1. Stroke. 2003. PMID: 12730558
-
Pyridoxalphosphate-6-azophenyl-2',4'-disulphonic acid (PPADS) as a tool for differentiation of P2Y2-receptor-mediated vasorelaxation in guinea pig aorta.Methods Find Exp Clin Pharmacol. 2002 Jul-Aug;24(6):351-6. doi: 10.1358/mf.2002.24.6.693067. Methods Find Exp Clin Pharmacol. 2002. PMID: 12224441
-
Endothelial P2-purinoceptors: subtypes and signal transduction.J Auton Pharmacol. 1996 Dec;16(6):353-6. doi: 10.1111/j.1474-8673.1996.tb00052.x. J Auton Pharmacol. 1996. PMID: 9131415 Review.
-
Ribose modified nucleosides and nucleotides as ligands for purine receptors.Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):333-41. doi: 10.1081/NCN-100002305. Nucleosides Nucleotides Nucleic Acids. 2001. PMID: 11563046 Free PMC article. Review.
Cited by
-
Synthesis and biological evaluation of sulfamoyl benzamide derivatives as selective inhibitors for h-NTPDases.RSC Adv. 2023 Jul 11;13(30):20909-20915. doi: 10.1039/d3ra03874b. eCollection 2023 Jul 7. RSC Adv. 2023. PMID: 37441049 Free PMC article.
-
NTPDase1/CD39 Ectonucleotidase Is Necessary for Normal Arterial Diameter Adaptation to Flow.Int J Mol Sci. 2023 Oct 10;24(20):15038. doi: 10.3390/ijms242015038. Int J Mol Sci. 2023. PMID: 37894719 Free PMC article.
-
The Interplay of Endothelial P2Y Receptors in Cardiovascular Health: From Vascular Physiology to Pathology.Int J Mol Sci. 2022 May 24;23(11):5883. doi: 10.3390/ijms23115883. Int J Mol Sci. 2022. PMID: 35682562 Free PMC article. Review.
-
The role of purinergic signaling in the etiology of migraine and novel antimigraine treatment.Purinergic Signal. 2015 Sep;11(3):307-16. doi: 10.1007/s11302-015-9453-8. Epub 2015 May 10. Purinergic Signal. 2015. PMID: 25957584 Free PMC article. Review.
-
Ecto-nucleoside triphosphate diphosphohydrolase 2 modulates local ATP-induced calcium signaling in human HaCaT keratinocytes.PLoS One. 2013;8(3):e57666. doi: 10.1371/journal.pone.0057666. Epub 2013 Mar 11. PLoS One. 2013. PMID: 23536768 Free PMC article.
References
-
- Ahmad S, Ahmad A, Ghosh M, Leslie CC, White CW. Extracellular ATP-mediated signaling for survival in hyperoxia-induced oxidative stress. J Biol Chem. 2004;279(16):16317–16325. - PubMed
-
- Ardlie NG, Perry DW, Packham MA, Mustard JF. Influence of apyrase on stability of suspensions of washed rabbit platelets. Proc Soc Exp Biol Med. 1971;136(4):1021–1023. - PubMed
-
- Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, et al. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74(3):777–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

