Production of interferon-gamma by activated T-cell receptor-alphabeta CD8alphabeta intestinal intraepithelial lymphocytes is required and sufficient for disruption of the intestinal barrier integrity
- PMID: 20067535
- PMCID: PMC2770683
- DOI: 10.1111/j.1365-2567.2009.03110.x
Production of interferon-gamma by activated T-cell receptor-alphabeta CD8alphabeta intestinal intraepithelial lymphocytes is required and sufficient for disruption of the intestinal barrier integrity
Abstract
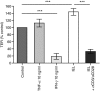
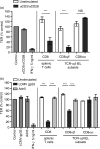
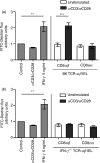
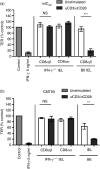
Maintenance of intestinal epithelial barrier function is of vital importance in preventing uncontrolled influx of antigens and the potentially ensuing inflammatory disorders. Intestinal intraepithelial lymphocytes (IEL) are in intimate contact with epithelial cells and may critically regulate the epithelial barrier integrity. While a preserving impact has been ascribed to the T-cell receptor (TCR)-gammadelta subset of IEL, IEL have also been shown to attenuate the barrier function. The present study sought to clarify the effects of IEL by specifically investigating the influence of the TCR-alphabeta CD8alphabeta and TCR-alphabeta CD8alphaalpha subsets of IEL on the intestinal epithelial barrier integrity. To this end, an in vitro coculture system of the murine intestinal crypt-derived cell-line mIC(cl2) and syngeneic ex vivo isolated IEL was employed. Epithelial integrity was assessed by analysis of transepithelial resistance (TER) and paracellular flux of fluorescein isothiocyanate-conjugated (FITC-) dextran. The TCR-alphabeta CD8alphaalpha IEL and resting TCR-alphabeta CD8alphabeta IEL did not affect TER of mIC(cl2) or flux of FITC-dextran. In contrast, activated TCR-alphabeta CD8alphabeta IEL clearly disrupted the integrity of the mIC(cl2) monolayer. No disrupting effect was seen with activated TCR-alphabeta CD8alphabeta IEL from interferon-gamma knockout mice. These findings demonstrate that secretion of interferon-gamma by activated TCR-alphabeta CD8alphabeta IEL is strictly required and also sufficient for disrupting the intestinal epithelial barrier function.
Figures

Similar articles
-
Enhanced differentiation of intraepithelial lymphocytes in the intestine of polymeric immunoglobulin receptor-deficient mice.Immunology. 2015 Sep;146(1):59-69. doi: 10.1111/imm.12480. Epub 2015 Jun 26. Immunology. 2015. PMID: 25967857 Free PMC article.
-
MHC class I allele dosage alters CD8 expression by intestinal intraepithelial lymphocytes.J Immunol. 2001 Sep 1;167(5):2561-8. doi: 10.4049/jimmunol.167.5.2561. J Immunol. 2001. PMID: 11509596
-
Late postnatal expansion of self-reactive CD8alphaalpha+ intestinal intraepithelial lymphocytes in mice.Autoimmunity. 2004 Dec;37(8):537-47. doi: 10.1080/08916930400027094. Autoimmunity. 2004. PMID: 15763916
-
The interaction of intestinal epithelial cells and intraepithelial lymphocytes in host defense.Immunol Res. 1999;20(3):219-35. doi: 10.1007/BF02790405. Immunol Res. 1999. PMID: 10741862 Review.
-
[T gamma-delta lymphocytes and their role in hypersensitivity processes in the digestive and respiratory mucosa].Allergol Immunopathol (Madr). 2002 Sep-Oct;30(5):273-82. Allergol Immunopathol (Madr). 2002. PMID: 12396962 Review. Spanish.
Cited by
-
Intraepithelial lymphocytes: to serve and protect.Curr Gastroenterol Rep. 2010 Dec;12(6):513-21. doi: 10.1007/s11894-010-0148-6. Curr Gastroenterol Rep. 2010. PMID: 20890736 Free PMC article. Review.
-
Single nucleotide polymorphisms within interferon signaling pathway genes are associated with colorectal cancer susceptibility and survival.PLoS One. 2014 Oct 28;9(10):e111061. doi: 10.1371/journal.pone.0111061. eCollection 2014. PLoS One. 2014. PMID: 25350395 Free PMC article.
-
Human Leukocyte Antigen (HLA) Haplotype Does Not Influence the Inflammatory Pattern of Duodenal Lymphocytosis Linked to Irritable Bowel Syndrome.Medicina (Kaunas). 2020 Nov 29;56(12):660. doi: 10.3390/medicina56120660. Medicina (Kaunas). 2020. PMID: 33260434 Free PMC article.
-
Enhanced differentiation of intraepithelial lymphocytes in the intestine of polymeric immunoglobulin receptor-deficient mice.Immunology. 2015 Sep;146(1):59-69. doi: 10.1111/imm.12480. Epub 2015 Jun 26. Immunology. 2015. PMID: 25967857 Free PMC article.
-
Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis.Sci Rep. 2018 Sep 21;8(1):14184. doi: 10.1038/s41598-018-32366-6. Sci Rep. 2018. PMID: 30242285 Free PMC article.
References
-
- McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–13. - PubMed
-
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. - PubMed
-
- Williams IR. Chemokine receptors and leukocyte trafficking in the mucosal immune system. Immunol Res. 2004;3:283–92. - PubMed
-
- Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells – polarity and complexity. Immunol Today. 2000;21:123–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

