Vaccinia virus decreases major histocompatibility complex (MHC) class II antigen presentation, T-cell priming, and peptide association with MHC class II
- PMID: 20067538
- PMCID: PMC2770686
- DOI: 10.1111/j.1365-2567.2009.03120.x
Vaccinia virus decreases major histocompatibility complex (MHC) class II antigen presentation, T-cell priming, and peptide association with MHC class II
Abstract
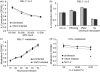
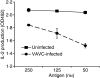
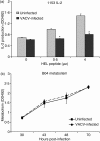
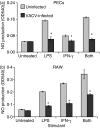
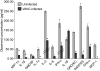
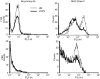
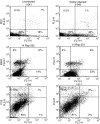
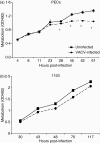
Vaccinia virus (VACV) is the current live virus vaccine used to protect humans against smallpox and monkeypox, but its use is contraindicated in several populations because of its virulence. It is therefore important to elucidate the immune evasion mechanisms of VACV. We found that VACV infection of antigen-presenting cells (APCs) significantly decreased major histocompatibility complex (MHC) II antigen presentation and decreased synthesis of 13 chemokines and cytokines, suggesting a potent viral mechanism for immune evasion. In these model systems, responding T cells were not directly affected by virus, indicating that VACV directly affects the APC. VACV significantly decreased nitric oxide production by peritoneal exudate cells and the RAW macrophage cell line in response to lipopolysaccharide (LPS) and interferon (IFN)-gamma, decreased class II MHC expression on APCs, and induced apoptosis in macrophages and dendritic cells. However, VACV decreased antigen presentation by 1153 B cells without apparent apoptosis induction, indicating that VACV differentially affects B lymphocytes and other APCs. We show that the key mechanism of VACV inhibition of antigen presentation may be its reduction of antigenic peptide loaded into the cleft of MHC class II molecules. These data indicate that VACV evades the host immune response by impairing critical functions of the APC.
Figures


Similar articles
-
Selective reconstitution of IFN‑γ gene function in Ncr1+ NK cells is sufficient to control systemic vaccinia virus infection.PLoS Pathog. 2020 Feb 5;16(2):e1008279. doi: 10.1371/journal.ppat.1008279. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32023327 Free PMC article.
-
Vaccinia virus blocks Stat1-dependent and Stat1-independent gene expression induced by type I and type II interferons.J Interferon Cytokine Res. 2008 Jun;28(6):367-80. doi: 10.1089/jir.2007.0113. J Interferon Cytokine Res. 2008. PMID: 18593332 Free PMC article.
-
The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice.J Clin Invest. 2012 May;122(5):1628-43. doi: 10.1172/JCI60660. Epub 2012 Apr 16. J Clin Invest. 2012. PMID: 22505455 Free PMC article.
-
Bacterial antigen delivery systems: phagocytic processing of bacterial antigens for MHC-I and MHC-II presentation to T cells.Behring Inst Mitt. 1997 Feb;(98):197-211. Behring Inst Mitt. 1997. PMID: 9382741 Review.
-
The ins and outs of MHC class II-mediated antigen processing and presentation.Nat Rev Immunol. 2015 Apr;15(4):203-16. doi: 10.1038/nri3818. Epub 2015 Feb 27. Nat Rev Immunol. 2015. PMID: 25720354 Free PMC article. Review.
Cited by
-
Factors Influencing Vaccine Hesitancy in China: A Qualitative Study.Vaccines (Basel). 2021 Nov 7;9(11):1291. doi: 10.3390/vaccines9111291. Vaccines (Basel). 2021. PMID: 34835221 Free PMC article.
-
The unique immune evasion mechanisms of the mpox virus and their implication for developing new vaccines and immunotherapies.Virol Sin. 2024 Oct;39(5):709-718. doi: 10.1016/j.virs.2024.08.008. Epub 2024 Aug 22. Virol Sin. 2024. PMID: 39181538 Free PMC article. Review.
-
Enhancing the Protective Immune Response to Administration of a LIVP-GFP Live Attenuated Vaccinia Virus to Mice.Pathogens. 2021 Mar 21;10(3):377. doi: 10.3390/pathogens10030377. Pathogens. 2021. PMID: 33801026 Free PMC article.
-
Selective reconstitution of IFN‑γ gene function in Ncr1+ NK cells is sufficient to control systemic vaccinia virus infection.PLoS Pathog. 2020 Feb 5;16(2):e1008279. doi: 10.1371/journal.ppat.1008279. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32023327 Free PMC article.
-
Immune modulation in primary vaccinia virus zoonotic human infections.Clin Dev Immunol. 2012;2012:974067. doi: 10.1155/2012/974067. Epub 2011 Dec 20. Clin Dev Immunol. 2012. PMID: 22229039 Free PMC article.
References
-
- Moss B. Poxviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2849–84.
-
- Mahalingam S, Damon IK, Lidbury BA. 25 years since the eradication of smallpox: why poxvirus research is still relevant. Trends Immunol. 2004;25:636–9. - PubMed
-
- Damaso CR, Esposito JJ, Condit RC, Moussatche N. An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virol. 2000;277:439–49. - PubMed
-
- Dhar AD, Werchniak AE, Li Y, Brennick JB, Goldsmith CS, Kline R, Damon I, Klaus SN. Tanapox infection in a college student. N Engl J Med. 2004;350:361–6. - PubMed
-
- Stich A, Meyer H, Kohler B, Fleischer K. Tanapox: first report in a European traveller and identification by PCR. Trans R Soc Trop Med Hyg. 2002;96:178–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

