MyD88 and interferon-alpha/beta are differentially required for dendritic cell maturation but dispensable for development of protective memory against Listeria
- PMID: 20067542
- PMCID: PMC2770690
- DOI: 10.1111/j.1365-2567.2009.03128.x
MyD88 and interferon-alpha/beta are differentially required for dendritic cell maturation but dispensable for development of protective memory against Listeria
Abstract
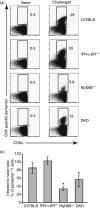
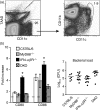
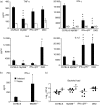
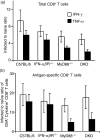
Signalling pathways mediated by MyD88 are important for sensing Toll-like receptor (TLR) ligands and directing an immune response. However, the influence of MyD88-derived cytokines and interferon (IFN)-alpha/beta, the latter being made by both MyD88-dependent and -independent pathways, in phenotypic and functional dendritic cell (DC) maturation during infection is poorly understood. Here we investigate the contribution of MyD88-dependent and -independent pathways to DC maturation, CD8 T-cell activation and the generation of protective memory against Listeria monocytogenes. We show that neither MyD88 deficiency alone nor MyD88/IFN-alphabetaR double deficiency alters Listeria-induced costimulatory molecule up-regulation on DCs in vivo. In contrast, DCs from infected IFN-alphabetaR(-/-) mice had higher CD80 and CD86 expression than wild-type DCs. We then examined the function of DCs matured in infected knockout mice. We found that DCs from Listeria-infected MyD88(-/-) and MyD88(-/-) IFN-alphabetaR(-/-) mice induced little or no IFN-gamma by CD8 T cells, respectively. In contrast, DCs from infected IFN-alphabetaR(-/-) mice had a greater capacity to induce IFN-gamma compared with DCs from infected wild-type mice. When the CD8 T-cell memory response was analysed, infected MyD88(-/-) and MyD88(-/- )IFN-alphabetaR(-/-) mice were found to have fewer bacteria-specific memory CD8 T cells than wild-type mice. However, the fraction of bacteria-specific CD8 T cells making IFN-gamma was similar in all mouse strains, and MyD88(-/-) and MyD88(-/- )IFN-alphabetaR(-/-) mice survived lethal challenge. Together the data suggest an inhibitory effect of IFN-alpha/beta on functional DC maturation during Listeria infection and reveal overlapping roles of MyD88-induced cytokines and IFN-alpha/beta in DC maturation and protective anti-Listeria immunity.
Figures


Similar articles
-
Plasmacytoid dendritic cells mature independently of MyD88 and IFN-αβR in response to Listeria and stimulate CD8 T cells.Immunol Lett. 2011 Aug 30;138(2):104-12. doi: 10.1016/j.imlet.2011.03.007. Epub 2011 Mar 29. Immunol Lett. 2011. PMID: 21453725
-
MyD88 and IFN-alphabeta differentially control maturation of bystander but not Salmonella-associated dendritic cells or CD11cintCD11b+ cells during infection.Cell Microbiol. 2008 Jul;10(7):1517-29. doi: 10.1111/j.1462-5822.2008.01144.x. Epub 2008 Mar 18. Cell Microbiol. 2008. PMID: 18363877
-
A role for IFN-gamma from antigen-specific CD8+ T cells in protective immunity to Listeria monocytogenes.J Immunol. 2007 Aug 15;179(4):2457-66. doi: 10.4049/jimmunol.179.4.2457. J Immunol. 2007. PMID: 17675507
-
Regulation of effector and memory T-cell functions by type I interferon.Immunology. 2011 Apr;132(4):466-74. doi: 10.1111/j.1365-2567.2011.03412.x. Epub 2011 Feb 14. Immunology. 2011. PMID: 21320124 Free PMC article. Review.
-
Insights into dendritic cell maturation during infection with application of advanced imaging techniques.Front Cell Infect Microbiol. 2023 Mar 2;13:1140765. doi: 10.3389/fcimb.2023.1140765. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36936763 Free PMC article. Review.
Cited by
-
Opportunities, Barriers, and a Strategy for Overcoming Translational Challenges to Therapeutic Nucleic Acid Nanotechnology.ACS Nano. 2020 Aug 25;14(8):9221-9227. doi: 10.1021/acsnano.0c04753. Epub 2020 Jul 24. ACS Nano. 2020. PMID: 32706238 Free PMC article.
-
Immune receptors involved in Streptococcus suis recognition by dendritic cells.PLoS One. 2012;7(9):e44746. doi: 10.1371/journal.pone.0044746. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984550 Free PMC article.
-
Listeria monocytogenes Inhibits Serotonin Transporter in Human Intestinal Caco-2 Cells.Microb Ecol. 2016 Oct;72(3):730-9. doi: 10.1007/s00248-016-0809-6. Epub 2016 Aug 3. Microb Ecol. 2016. PMID: 27488594 Free PMC article.
-
Early events regulating immunity and pathogenesis during Listeria monocytogenes infection.Trends Immunol. 2012 Oct;33(10):488-95. doi: 10.1016/j.it.2012.04.007. Epub 2012 Jun 5. Trends Immunol. 2012. PMID: 22677184 Free PMC article. Review.
-
IRF7 regulates TLR2-mediated activation of splenic CD11c(hi) dendritic cells.PLoS One. 2012;7(7):e41050. doi: 10.1371/journal.pone.0041050. Epub 2012 Jul 16. PLoS One. 2012. PMID: 22815909 Free PMC article.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
-
- Brewig N, Kissenpfennig A, Malissen B, Veit A, Bickert T, Fleischer B, Mostbock S, Ritter U. Priming of CD8+ and CD4+ T cells in experimental leishmaniasis is initiated by different dendritic cell subtypes. J Immunol. 2009;182:774–83. - PubMed
-
- Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

