An examination of alpha B-crystallin as a modifier of SOD1 aggregate pathology and toxicity in models of familial amyotrophic lateral sclerosis
- PMID: 20067574
- PMCID: PMC3971727
- DOI: 10.1111/j.1471-4159.2010.06572.x
An examination of alpha B-crystallin as a modifier of SOD1 aggregate pathology and toxicity in models of familial amyotrophic lateral sclerosis
Abstract
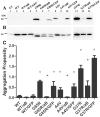
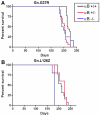
Amyotrophic lateral sclerosis is a progressively paralytic neurodegenerative disease that can be caused by mutations in Cu,Zn-superoxide dismutase 1 (SOD1). Transgenic mice that over-express mutant SOD1 develop paralysis and accumulate aggregates of mutant protein in the brainstem and spinal cord. The present study uses a cell culture model to demonstrate alpha B-crystallin is capable of reducing aggregation of mutant SOD1. To test the role of alpha B-crystallin in modulating SOD1 aggregation in vivo, alpha B-crystallin deficient mice were bred to mice expressing two different SOD1 mutants (G37R and L126Z). Although completely eliminating alpha B-crystallin reduced the interval to disease endstage by 20-30 days in mice expressing either mutant, there were no detectable changes in the levels of sedimentable, SOD1 aggregates in the spinal cord of symptomatic mice. Because alpha B-crystallin is most abundantly expressed in muscle, we expected that the loss of this chaperone would leave this tissue vulnerable to mutant SOD1 aggregation. However, there was no evidence of mutant SOD1 aggregation in the muscle of mice lacking alpha B-crystallin. Our findings indicate that a significant perturbation to the protein homeostasis network of muscle is not sufficient to induce the aggregation of misfolded mutant SOD1. These outcomes have implications regarding the role of chaperones in modulating the tissue specific accumulations of misfolded SOD1.
Figures



Similar articles
-
Substantially elevating the levels of αB-crystallin in spinal motor neurons of mutant SOD1 mice does not significantly delay paralysis or attenuate mutant protein aggregation.J Neurochem. 2015 May;133(3):452-64. doi: 10.1111/jnc.13022. Epub 2015 Jan 26. J Neurochem. 2015. PMID: 25557022 Free PMC article.
-
Somatodendritic accumulation of misfolded SOD1-L126Z in motor neurons mediates degeneration: alphaB-crystallin modulates aggregation.Hum Mol Genet. 2005 Aug 15;14(16):2335-47. doi: 10.1093/hmg/ddi236. Epub 2005 Jul 6. Hum Mol Genet. 2005. PMID: 16000321
-
Coincident thresholds of mutant protein for paralytic disease and protein aggregation caused by restrictively expressed superoxide dismutase cDNA.Neurobiol Dis. 2005 Dec;20(3):943-52. doi: 10.1016/j.nbd.2005.06.005. Epub 2005 Jul 19. Neurobiol Dis. 2005. PMID: 16046140
-
Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261
-
Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation.Neuropathology. 2001 Mar;21(1):82-92. doi: 10.1046/j.1440-1789.2001.00361.x. Neuropathology. 2001. PMID: 11304046 Review.
Cited by
-
An examination of wild-type SOD1 in modulating the toxicity and aggregation of ALS-associated mutant SOD1.Hum Mol Genet. 2010 Dec 15;19(24):4774-89. doi: 10.1093/hmg/ddq408. Epub 2010 Sep 24. Hum Mol Genet. 2010. PMID: 20871097 Free PMC article.
-
Analysis of chaperone mRNA expression in the adult mouse brain by meta analysis of the Allen Brain Atlas.PLoS One. 2010 Oct 28;5(10):e13675. doi: 10.1371/journal.pone.0013675. PLoS One. 2010. PMID: 21060842 Free PMC article.
-
Small heat-shock proteins: important players in regulating cellular proteostasis.Cell Mol Life Sci. 2015 Feb;72(3):429-451. doi: 10.1007/s00018-014-1754-5. Epub 2014 Oct 29. Cell Mol Life Sci. 2015. PMID: 25352169 Free PMC article. Review.
-
AAV2/9-mediated overexpression of MIF inhibits SOD1 misfolding, delays disease onset, and extends survival in mouse models of ALS.Proc Natl Acad Sci U S A. 2019 Jul 16;116(29):14755-14760. doi: 10.1073/pnas.1904665116. Epub 2019 Jul 1. Proc Natl Acad Sci U S A. 2019. PMID: 31262807 Free PMC article.
-
Superoxide dismutase 1 encoding mutations linked to ALS adopts a spectrum of misfolded states.Mol Neurodegener. 2011 Nov 17;6:77. doi: 10.1186/1750-1326-6-77. Mol Neurodegener. 2011. PMID: 22094223 Free PMC article.
References
-
- Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42:2924–2934. - PubMed
-
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. - PubMed
-
- Devlin GL, Carver JA, Bottomley SP. The selective inhibition of serpin aggregation by the molecular chaperone, alpha-crystallin, indicates a nucleation-dependent specificity. J Biol Chem. 2003;278: 48644–48650. - PubMed
-
- Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, Belia S, Wannenes F, Nicoletti C, Del Prete Z, Rosenthal N, Molinaro M, Protasi F, Fano G, Sandri M, Musaro A. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

