An examination of alpha B-crystallin as a modifier of SOD1 aggregate pathology and toxicity in models of familial amyotrophic lateral sclerosis
- PMID: 20067574
- PMCID: PMC3971727
- DOI: 10.1111/j.1471-4159.2010.06572.x
An examination of alpha B-crystallin as a modifier of SOD1 aggregate pathology and toxicity in models of familial amyotrophic lateral sclerosis
Abstract
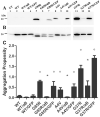
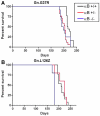
Amyotrophic lateral sclerosis is a progressively paralytic neurodegenerative disease that can be caused by mutations in Cu,Zn-superoxide dismutase 1 (SOD1). Transgenic mice that over-express mutant SOD1 develop paralysis and accumulate aggregates of mutant protein in the brainstem and spinal cord. The present study uses a cell culture model to demonstrate alpha B-crystallin is capable of reducing aggregation of mutant SOD1. To test the role of alpha B-crystallin in modulating SOD1 aggregation in vivo, alpha B-crystallin deficient mice were bred to mice expressing two different SOD1 mutants (G37R and L126Z). Although completely eliminating alpha B-crystallin reduced the interval to disease endstage by 20-30 days in mice expressing either mutant, there were no detectable changes in the levels of sedimentable, SOD1 aggregates in the spinal cord of symptomatic mice. Because alpha B-crystallin is most abundantly expressed in muscle, we expected that the loss of this chaperone would leave this tissue vulnerable to mutant SOD1 aggregation. However, there was no evidence of mutant SOD1 aggregation in the muscle of mice lacking alpha B-crystallin. Our findings indicate that a significant perturbation to the protein homeostasis network of muscle is not sufficient to induce the aggregation of misfolded mutant SOD1. These outcomes have implications regarding the role of chaperones in modulating the tissue specific accumulations of misfolded SOD1.
Figures



References
-
- Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42:2924–2934. - PubMed
-
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. - PubMed
-
- Devlin GL, Carver JA, Bottomley SP. The selective inhibition of serpin aggregation by the molecular chaperone, alpha-crystallin, indicates a nucleation-dependent specificity. J Biol Chem. 2003;278: 48644–48650. - PubMed
-
- Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, Belia S, Wannenes F, Nicoletti C, Del Prete Z, Rosenthal N, Molinaro M, Protasi F, Fano G, Sandri M, Musaro A. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

