Hydrogen sulfide as a gasotransmitter
- PMID: 20067586
- PMCID: PMC2965526
- DOI: 10.1111/j.1471-4159.2010.06580.x
Hydrogen sulfide as a gasotransmitter
Abstract
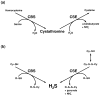
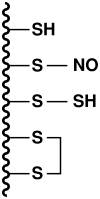
Nitric oxide (NO) and carbon monoxide (CO) are well established as messenger molecules throughout the body, gasotransmitters, based on striking alterations in mice lacking the appropriate biosynthetic enzymes. Hydrogen sulfide (H(2)S) is even more chemically reactive, but until recently there was little definitive evidence for its physiologic formation. Cystathionine beta-synthase (EC 4.2.1.22), and cystathionine gamma-lyase (CSE; EC 4.4.1.1), also known as cystathionine, can generate H(2)S from cyst(e)ine. Very recent studies with mice lacking these enzymes have established that CSE is responsible for H(2)S formation in the periphery, while in the brain cystathionine beta-synthase is the biosynthetic enzyme. Endothelial-derived relaxing factor activity is reduced 80% in the mesenteric artery of mice with deletion of CSE, establishing H(2)S as a major physiologic endothelial-derived relaxing factor. H(2)S appears to signal predominantly by S-sulfhydrating cysteines in its target proteins, analogous to S-nitrosylation by NO. Whereas S-nitrosylation typically inhibits enzymes, S-sulfhydration activates them. S-nitrosylation basally affects 1-2% of its target proteins, while 10-25% of H(2)S target proteins are S-sulfhydrated. In summary, H(2)S appears to be a physiologic gasotransmitter of comparable importance to NO and carbon monoxide.
Keywords: EDHF; EDRF; GAPDH; KATP; S-sulfhydration; cystathionase; cystathionine β-synthase; cystathionine γ-lyase; hydrogen sulfide; hydropersulfide.
Figures

References
-
- Abeles RH, Walsh CT. Acetylenic enzyme inactivators. Inactivation of γ-cystathionase, in vitro and in vivo, by propargyl glycine. J Am Chem Soc. 1973;95:6124–6125. - PubMed
-
- Abott MH, Folstein SE, Abbey H, Pyeritz RE. Psychiatric manifestations of homocystinuria due to cystathionine β-synthase deficiency: Prevalence, natural history, and relationship to neurologic impairment and vitamin B6-responsiveness. Am J Med Genet. 1987;26:959–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

