Effects of autonomic nerve stimulation on colorectal motility in rats
- PMID: 20067587
- PMCID: PMC2952396
- DOI: 10.1111/j.1365-2982.2009.01461.x
Effects of autonomic nerve stimulation on colorectal motility in rats
Abstract
Background: Several disease processes of the colon and rectum, including constipation and incontinence, have been associated with abnormalities of the autonomic nervous system. However, the autonomic innervation to the colon and rectum are not fully understood. The aims of this study were to investigate the effect of stimulation of vagus nerves, pelvic nerves (PN) and hypogastric nerves (HGN) on colorectal motility in rats.
Methods: Four strain gauge transducers were implanted on the proximal colon, mid colon, distal colon and rectum to record circular muscle contractions in rats. Electrical stimulation was administered to the efferent distal ends of the cervical vagus nerve, PN and HGN. Motility index (MI) was evaluated before and during stimulation.
Key results: Electrical stimulation (5-20 Hz) of the cervical vagus elicited significant contractions in the mid colon and distal colon, whereas less pronounced contractions were observed in the proximal colon. Pelvic nerves stimulation elicited significant contractions in the rectum as well as the mid colon and distal colon. Atropine treatment almost completely abolished the contractions induced by vagus nerve and PN stimulation. Hypogastric nerves stimulation caused relaxations in the rectum, mid colon and distal colon. The relaxations in response to HGN stimulation were abolished by propranolol.
Conclusions & inferences: Vagal innervation extends to the distal colon, while the PN has projections in the distribution of the rectum through the mid colon. This suggests a pattern of dual parasympathetic innervation in the left colon. Parasympathetic fibers regulate colorectal contractions via muscarinic receptors. The HGN mainly regulates colorectal relaxations via beta-adrenoceptors.
Conflict of interest statement
No conflicts of interest exist
Figures
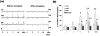


Similar articles
-
Sympathetic and parasympathetic regulation of rectal motility in rats.J Gastrointest Surg. 2009 Nov;13(11):2027-33; discussion 2033. doi: 10.1007/s11605-009-0999-z. Epub 2009 Sep 16. J Gastrointest Surg. 2009. PMID: 19760300
-
The role of the rectal branches of pelvic plexus in defecation and colonic motility in a canine model.J Med Dent Sci. 2003 Dec;50(4):275-84. J Med Dent Sci. 2003. PMID: 15074355
-
Ano-rectal motility responses to pelvic, hypogastric and pudendal nerve stimulation in the Göttingen minipig.Neurogastroenterol Motil. 2006 Feb;18(2):153-61. doi: 10.1111/j.1365-2982.2005.00735.x. Neurogastroenterol Motil. 2006. PMID: 16420294
-
Autonomic influences on colorectal motility and pelvic surgery.World J Surg. 1992 Sep-Oct;16(5):811-9. doi: 10.1007/BF02066975. World J Surg. 1992. PMID: 1462613
-
Physiology and pathophysiology of colonic motor activity (2).Dig Dis Sci. 1991 Jul;36(7):998-1018. doi: 10.1007/BF01297155. Dig Dis Sci. 1991. PMID: 2070711 Review.
Cited by
-
The effect of colonic tissue electrical stimulation and celiac branch of the abdominal vagus nerve neuromodulation on colonic motility in anesthetized pigs.Neurogastroenterol Motil. 2020 Nov;32(11):e13925. doi: 10.1111/nmo.13925. Epub 2020 Jun 23. Neurogastroenterol Motil. 2020. PMID: 32578346 Free PMC article.
-
Colonic electrical stimulation for the treatment of slow-transit constipation: a preliminary pilot study.Surg Endosc. 2014 Feb;28(2):691-7. doi: 10.1007/s00464-013-3192-0. Epub 2013 Sep 19. Surg Endosc. 2014. PMID: 24048815
-
Control of colonic motility using electrical stimulation to modulate enteric neural activity.Am J Physiol Gastrointest Liver Physiol. 2021 Apr 1;320(4):G675-G687. doi: 10.1152/ajpgi.00463.2020. Epub 2021 Feb 24. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33624530 Free PMC article.
-
Distinct projection targets define subpopulations of mouse brainstem vagal neurons that express the autism-associated MET receptor tyrosine kinase.J Comp Neurol. 2017 Dec 15;525(18):3787-3808. doi: 10.1002/cne.24294. Epub 2017 Aug 11. J Comp Neurol. 2017. PMID: 28758209 Free PMC article.
-
Tonic contraction develops in the colon during anaphylactic hypotension in anesthetized rats.J Physiol Sci. 2019 Nov;69(6):953-960. doi: 10.1007/s12576-019-00710-8. Epub 2019 Sep 21. J Physiol Sci. 2019. PMID: 31542858 Free PMC article.
References
-
- Mochiki E, Asao T, Kuwano H. Gastrointestinal motility after digestive surgery. Surg Today. 2007;37(12):1023–32. - PubMed
-
- Iizuka I, Koda K, Seike K, et al. Defecatory malfunction caused by motility disorder of the neorectum after anterior resection for rectal cancer. Am J Surg. 2004;188(2):176–80. - PubMed
-
- Dorofeeva AA, Panteleev SS, Makarov FN. Parasympathetic innervation of the initial segments of the large intestine in cats. Neurosci Behav Physiol. 2008;38(9):923–7. - PubMed
-
- Shimizu K, Koda K, Kase Y, et al. Induction and recovery of colonic motility/defecatory disorders after extrinsic denervation of the colon and rectum in rats. Surgery. 2006;139(3):395–406. - PubMed
-
- Devroede G, Lamarche J. Functional importance of extrinsic parasympathetic innervation to the distal colon and rectum in man. Gastroenterology. 1974;66(2):273–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

