Smoothened as a new therapeutic target for human osteosarcoma
- PMID: 20067614
- PMCID: PMC2818696
- DOI: 10.1186/1476-4598-9-5
Smoothened as a new therapeutic target for human osteosarcoma
Abstract
Background: The Hedgehog signaling pathway functions as an organizer in embryonic development. Recent studies have demonstrated constitutive activation of Hedgehog pathway in various types of malignancies. However, it remains unclear how Hedgehog pathway is involved in the pathogenesis of osteosarcoma. To explore the involvement of aberrant Hedgehog pathway in the pathogenesis of osteosarcoma, we investigated the expression and activation of Hedgehog pathway in osteosarcoma and examined the effect of SMOOTHENED (SMO) inhibition.
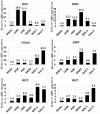
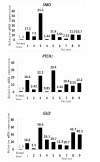
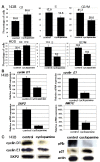
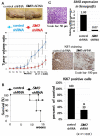
Results: To evaluate the expression of genes of Hedgehog pathway, we performed real-time PCR and immunohistochemistry using osteosarcoma cell lines and osteosarcoma biopsy specimens. To evaluate the effect of SMO inhibition, we did cell viability, colony formation, cell cycle in vitro and xenograft model in vivo. Real-time PCR revealed that osteosarcoma cell lines over-expressed Sonic hedgehog, Indian hedgehog, PTCH1, SMO, and GLI. Real-time PCR revealed over-expression of SMO, PTCH1, and GLI2 in osteosarcoma biopsy specimens. These findings showed that Hedgehog pathway is activated in osteosarcomas. Inhibition of SMO by cyclopamine, a specific inhibitor of SMO, slowed the growth of osteosarcoma in vitro. Cell cycle analysis revealed that cyclopamine promoted G1 arrest. Cyclopamine reduced the expression of accelerators of the cell cycle including cyclin D1, cyclin E1, SKP2, and pRb. On the other hand, p21(cip1) wprotein was up-regulated by cyclopamine treatment. In addition, knockdown of SMO by SMO shRNA prevents osteosarcoma growth in vitro and in vivo.
Conclusions: These findings suggest that inactivation of SMO may be a useful approach to the treatment of patients with osteosarcoma.
Figures


References
-
- Gibbs CP Jr, Weber K, Scarborough MT. Malignant bone tumors. Instr Course Lect. 2002;51:413–428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

