Metabolic engineering of Agrobacterium sp. strain ATCC 31749 for production of an alpha-Gal epitope
- PMID: 20067629
- PMCID: PMC2818619
- DOI: 10.1186/1475-2859-9-1
Metabolic engineering of Agrobacterium sp. strain ATCC 31749 for production of an alpha-Gal epitope
Abstract
Background: Oligosaccharides containing a terminal Gal-alpha1,3-Gal moiety are collectively known as alpha-Gal epitopes. alpha-Gal epitopes are integral components of several medical treatments under development, including flu and HIV vaccines as well as cancer treatments. The difficulty associated with synthesizing the alpha-Gal epitope hinders the development and application of these treatments due to the limited availability and high cost of the alpha-Gal epitope. This work illustrates the development of a whole-cell biocatalyst for synthesizing the alpha-Gal epitope, Gal-alpha1,3-Lac.
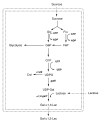
Results: Agrobacterium sp. ATCC 31749 was engineered to produce Gal-alpha1,3-Lac by the introduction of a UDP-galactose 4'-epimerase:alpha1,3-galactosyltransferase fusion enzyme. The engineered Agrobacterium synthesized 0.4 g/L of the alpha-Gal epitope. Additional metabolic engineering efforts addressed the factors limiting alpha-Gal epitope production, namely the availability of the two substrates, lactose and UDP-glucose. Through expression of a lactose permease, the intracellular lactose concentration increased by 60 to 110%, subsequently leading to an improvement in Gal-alpha1,3-Lac production. Knockout of the curdlan synthase gene increased UDP-glucose availability by eliminating the consumption of UDP-glucose for synthesis of the curdlan polysaccharide. With these additional engineering efforts, the final engineered strain synthesized approximately 1 g/L of Gal-alpha1,3-Lac.
Conclusions: The Agrobacterium biocatalyst developed in this work synthesizes gram-scale quantities of alpha-Gal epitope and does not require expensive cofactors or permeabilization, making it a useful biocatalyst for industrial production of the alpha-Gal epitope. Furthermore, the engineered Agrobacterium, with increased lactose uptake and improved UDP-glucose availability, is a promising host for the production of other medically-relevant oligosaccharides.
Figures





Similar articles
-
Changing the donor cofactor of bovine alpha 1, 3-galactosyltransferase by fusion with UDP-galactose 4-epimerase. More efficient biocatalysis for synthesis of alpha-Gal epitopes.J Biol Chem. 2000 Oct 13;275(41):31594-600. doi: 10.1074/jbc.M004005200. J Biol Chem. 2000. PMID: 10913140
-
Metabolic engineering of Agrobacterium sp. for UDP-galactose regeneration and oligosaccharide synthesis.Metab Eng. 2006 Sep;8(5):465-73. doi: 10.1016/j.ymben.2006.05.004. Epub 2006 Jun 7. Metab Eng. 2006. PMID: 16890004
-
Production of alpha-galactosyl epitopes via combined use of two recombinant whole cells harboring UDP-galactose 4-epimerase and alpha-1,3-galactosyltransferase.Biotechnol Prog. 2000 Jul-Aug;16(4):595-9. doi: 10.1021/bp000052s. Biotechnol Prog. 2000. PMID: 10933834
-
Characteristics of α-Gal epitope, anti-Gal antibody, α1,3 galactosyltransferase and its clinical exploitation (Review).Int J Mol Med. 2016 Jan;37(1):11-20. doi: 10.3892/ijmm.2015.2397. Epub 2015 Oct 30. Int J Mol Med. 2016. PMID: 26531137 Free PMC article. Review.
-
The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy.Immunol Cell Biol. 2005 Dec;83(6):674-86. doi: 10.1111/j.1440-1711.2005.01366.x. Immunol Cell Biol. 2005. PMID: 16266320 Review.
Cited by
-
Cell-Based Meat Safety and Regulatory Approaches: A Comprehensive Review.Food Sci Anim Resour. 2025 Jan;45(1):145-164. doi: 10.5851/kosfa.2024.e122. Epub 2025 Jan 1. Food Sci Anim Resour. 2025. PMID: 39840246 Free PMC article. Review.
-
Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies.Front Microbiol. 2015 May 26;6:496. doi: 10.3389/fmicb.2015.00496. eCollection 2015. Front Microbiol. 2015. PMID: 26074894 Free PMC article. Review.
-
Alleviation of Oxidative Damage Induced by CaCl2 Priming Is Related to Osmotic and Ion Stress Reduction Rather Than Enhanced Antioxidant Capacity During Germination Under Salt Stress in Sorghum.Front Plant Sci. 2022 Apr 29;13:881039. doi: 10.3389/fpls.2022.881039. eCollection 2022. Front Plant Sci. 2022. PMID: 35574088 Free PMC article.
-
The sweet branch of metabolic engineering: cherry-picking the low-hanging sugary fruits.Microb Cell Fact. 2015 Dec 9;14:197. doi: 10.1186/s12934-015-0389-z. Microb Cell Fact. 2015. PMID: 26655367 Free PMC article. Review.
-
Metabolic engineering of Agrobacterium sp. ATCC31749 for curdlan production from cellobiose.J Ind Microbiol Biotechnol. 2016 Sep;43(9):1323-31. doi: 10.1007/s10295-016-1805-z. Epub 2016 Jul 8. J Ind Microbiol Biotechnol. 2016. PMID: 27387419
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

