Lymph heart musculature is under distinct developmental control from lymphatic endothelium
- PMID: 20067786
- PMCID: PMC2845526
- DOI: 10.1016/j.ydbio.2010.01.002
Lymph heart musculature is under distinct developmental control from lymphatic endothelium
Abstract
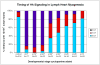
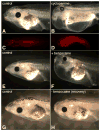
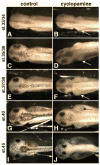
Lymph hearts are pulsatile organs, present in lower vertebrates, that function to propel lymph into the venous system. Although they are absent in mammals, the initial veno-lymphatic plexus that forms during mammalian jugular lymph sac development has been described as the vestigial homologue of the nascent stage of ancestral anterior lymph hearts. Despite the widespread presence of lymph hearts among vertebrate species and their unique function, extremely little is known about lymph heart development. We show that Xenopus anterior lymph heart muscle expresses skeletal muscle markers such as myoD and 12/101, rather than cardiac markers. The onset of lymph heart myoblast induction can be visualized by engrailed-1 (en1) staining in anterior trunk somites, which is dependent on Hedgehog (Hh) signaling. In the absence of Hh signaling and upon en1 knockdown, lymph heart muscle fails to develop, despite the normal development of the lymphatic endothelium of the lymph heart, and embryos develop edema. These results suggest a mechanism for the evolutionary transition from anterior lymph hearts to jugular lymph sacs in mammals.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Cheng L, Alvares LE, Ahmed MU, El-Hanfy AS, Dietrich S. The epaxial-hypaxial subdivision of the avian somite. Dev Biol. 2004;274:348–69. - PubMed
-
- Cleaver O, Tonissen KF, Saha MS, Krieg PA. Neovascularization of the Xenopus embryo. Dev Dyn. 1997;210:66–77. - PubMed
-
- Currie PD, Ingham PW. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature. 1996;382:452–5. - PubMed
-
- Davis CA, Holmyard DP, Millen KJ, Joyner AL. Examining pattern formation in mouse, chicken and frog embryos with an En-specific antiserum. Development. 1991;111:287–98. - PubMed
-
- Devic E, Paquereau L, Vernier P, Knibiehler B, Audigier Y. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech Dev. 1996;59:129–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

