Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome
- PMID: 20067811
- PMCID: PMC2847670
- DOI: 10.1016/j.jconrel.2009.12.031
Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome
Abstract
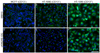
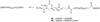
The Asn-Gly-Arg (NGR) motif in both cyclic and linear form has previously been shown to specifically bind to CD13/aminopeptidase N that is selectively overexpressed in tumor vasculature and some tumor cells. However, previous versions of cyclic NGR used a liable disulfide bridge between cysteine residues that may be problematic for liposome targeting due to disulfide bond formation between adjacent peptides on the liposomal surface. In this study, we report the design, synthesis, and characterization of a novel cyclic NGR-containing peptide, cKNGRE, which does not contain a disulfide bridge. cKNGRE was synthesized in good yield and purity and attached to the fluorescent reporter Oregon Green (cKNGRE-OG) and lysolipid-containing temperature sensitive liposomes (LTSLs). The identity of cKNGRE was verified with NMR and mass spectral techniques. In vitro fluorescence microscopy evaluation of cKNGRE-OG demonstrated binding and active uptake by CD13(+) cancer cells and minimal binding to CD13(-) cancer cells. The cKNGRE-OG ligand displayed 3.6-fold greater affinity for CD13(+) cancer cells than a linear NGR-containing peptide. Affinity for CD13(+) cancer cells was similarly improved 10-fold for both the cyclic and linear NGR when presented in a multivalent fashion on the surface of an LTSL. cKNGRE-targeted LTSLs rapidly released (>75% in <4s) doxorubicin at 41.3 degrees C with minimal release at 37 degrees C. These results demonstrate the ability to synthesize a cKNGRE-targeted temperature sensitive liposome that lacks a disulfide bridge and has sufficient binding affinity for biological applications.
Published by Elsevier B.V.
Figures


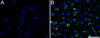






Similar articles
-
68Ga-labeled cyclic NGR peptide for microPET imaging of CD13 receptor expression.Molecules. 2014 Aug 5;19(8):11600-12. doi: 10.3390/molecules190811600. Molecules. 2014. PMID: 25100253 Free PMC article.
-
NGR peptide ligands for targeting CD13/APN identified through peptide array screening resemble fibronectin sequences.ACS Comb Sci. 2012 Nov 12;14(11):590-9. doi: 10.1021/co300055s. Epub 2012 Oct 11. ACS Comb Sci. 2012. PMID: 23030271
-
Critical role of flanking residues in NGR-to-isoDGR transition and CD13/integrin receptor switching.J Biol Chem. 2010 Mar 19;285(12):9114-23. doi: 10.1074/jbc.M109.044297. Epub 2010 Jan 11. J Biol Chem. 2010. PMID: 20064928 Free PMC article.
-
[Advance in studies on NGR peptide modified liposome and its anti-tumor performance].Zhongguo Zhong Yao Za Zhi. 2013 Jul;38(13):2041-5. Zhongguo Zhong Yao Za Zhi. 2013. PMID: 24079222 Review. Chinese.
-
The neovasculature homing motif NGR: more than meets the eye.Blood. 2008 Oct 1;112(7):2628-35. doi: 10.1182/blood-2008-04-150862. Epub 2008 Jun 23. Blood. 2008. PMID: 18574027 Free PMC article. Review.
Cited by
-
Effects of doxorubicin-encapsulating AG73 peptide-modified liposomes on tumor selectivity and cytotoxicity.Results Pharma Sci. 2011 Oct 13;1(1):68-75. doi: 10.1016/j.rinphs.2011.10.001. eCollection 2011 May. Results Pharma Sci. 2011. PMID: 25755984 Free PMC article.
-
Research Progress of Radiolabeled Asn-Gly-Arg (NGR) Peptides for Imaging and Therapy.Mol Imaging. 2020 Jan-Dec;19:1536012120934957. doi: 10.1177/1536012120934957. Mol Imaging. 2020. PMID: 32862776 Free PMC article. Review.
-
pH-sensitive, polymer modified, plasma stable niosomes: promising carriers for anti-cancer drugs.EXCLI J. 2015 Jan 6;14:21-32. doi: 10.17179/excli2013-609. eCollection 2015. EXCLI J. 2015. PMID: 26417350 Free PMC article.
-
Targeted polymeric therapeutic nanoparticles: design, development and clinical translation.Chem Soc Rev. 2012 Apr 7;41(7):2971-3010. doi: 10.1039/c2cs15344k. Epub 2012 Mar 5. Chem Soc Rev. 2012. PMID: 22388185 Free PMC article. Review.
-
In Vivo Tumor Angiogenesis Imaging Using Peptide-Based Near-Infrared Fluorescent Probes.Methods Mol Biol. 2016;1444:73-84. doi: 10.1007/978-1-4939-3721-9_8. Methods Mol Biol. 2016. PMID: 27283419 Free PMC article.
References
-
- Stevanovic S. Identification of tumour-associated T-CELL epitopes for vaccine development. Nature Reviews Cancer. 2002;2(7):514–520. - PubMed
-
- Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377–380. - PubMed
-
- Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nature Reviews Cancer. 2002;2(10):750–763. - PubMed
-
- Brignole C, Marimpietri D, Gambini C, Allen TM, Ponzoni M, Pastorino F. Development of Fab' fragments of anti-GD(2) immunoliposomes entrapping doxorubicin for experimental therapy of human neuroblastoma. Cancer Letters. 2003;197(1–2):199–204. - PubMed
-
- Pagnan G, Montaldo PC, Pastorino F, Raffaghello L, Kirchmeier M, Allen TM, Ponzoni M. GD2-mediated melanoma cell targeting and cytotoxicity of liposome-entrapped fenretinide. International Journal of Cancer. 1999;81(2):268–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

