Human embryonic stem cell-derived vascular progenitor cells capable of endothelial and smooth muscle cell function
- PMID: 20067819
- PMCID: PMC2838385
- DOI: 10.1016/j.exphem.2010.01.001
Human embryonic stem cell-derived vascular progenitor cells capable of endothelial and smooth muscle cell function
Abstract
Objective: Previous studies have demonstrated development of endothelial cells (ECs) and smooth muscle cells (SMCs) as separate cell lineages derived from human embryonic stem cells (hESCs). We demonstrate CD34(+) cells isolated from differentiated hESCs function as vascular progenitor cells capable of producing both ECs and SMCs. These studies better define the developmental origin and reveal the relationship between these two cell types, as well as provide a more complete biological characterization.
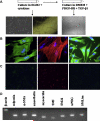
Materials and methods: hESCs are cocultured on M2-10B4 stromal cells or Wnt1-expressing M2-10B4 for 13 to 15 days to generate a CD34(+) cell population. These cells are isolated using a magnetic antibody separation kit and cultured on fibronectin-coated dishes in EC medium. To induce SMC differentiation, culture medium is changed and a morphological and phenotypic change occurs within 24 to 48 hours.
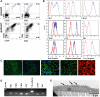
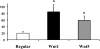
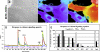
Results: CD34(+) vascular progenitor cells give rise to ECs and SMCs. The two populations express respective cell-specific transcripts and proteins, exhibit intracellular calcium in response to various agonists, and form robust tube-like structures when cocultured in Matrigel. Human umbilical vein endothelial cells cultured under SMC conditions do not exhibit a change in phenotype or genotype. Wnt1-overexpressing stromal cells produced an increased number of progenitor cells.
Conclusions: The ability to generate large numbers of ECs and SMCs from a single vascular progenitor cell population is promising for therapeutic use to treat a variety of diseased and ischemic conditions. The stepwise differentiation outlined here is an efficient, reproducible method with potential for large-scale cultures suitable for clinical applications.
Copyright 2010 ISEH - Society for Hematology and Stem Cells. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Endothelial progenitor cells derived from CD34+ cells form cooperative vascular networks.Cell Physiol Biochem. 2010;26(4-5):679-88. doi: 10.1159/000322335. Epub 2010 Oct 29. Cell Physiol Biochem. 2010. PMID: 21063105
-
Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo.Circ Res. 2007 Aug 3;101(3):286-94. doi: 10.1161/CIRCRESAHA.107.150201. Epub 2007 Jun 14. Circ Res. 2007. PMID: 17569886
-
Embryological Origin of Human Smooth Muscle Cells Influences Their Ability to Support Endothelial Network Formation.Stem Cells Transl Med. 2016 Jul;5(7):946-59. doi: 10.5966/sctm.2015-0282. Epub 2016 May 18. Stem Cells Transl Med. 2016. PMID: 27194743 Free PMC article.
-
Pluripotent stem cell differentiation into vascular cells: a novel technology with promises for vascular re(generation).Pharmacol Ther. 2011 Jan;129(1):29-49. doi: 10.1016/j.pharmthera.2010.10.004. Epub 2010 Oct 20. Pharmacol Ther. 2011. PMID: 20965210 Review.
-
Human embryonic stem cell-derived vascular smooth muscle cells in therapeutic neovascularisation.J Mol Cell Cardiol. 2011 Nov;51(5):651-64. doi: 10.1016/j.yjmcc.2011.07.014. Epub 2011 Jul 24. J Mol Cell Cardiol. 2011. PMID: 21816157 Review.
Cited by
-
Endothelial reconstitution by CD34+ progenitors derived from baboon embryonic stem cells.J Cell Mol Med. 2013 Feb;17(2):242-51. doi: 10.1111/jcmm.12002. Epub 2013 Jan 10. J Cell Mol Med. 2013. PMID: 23301772 Free PMC article.
-
New Solutions for Old Problems: How Reproductive Tissue Engineering Has Been Revolutionizing Reproductive Medicine.Ann Biomed Eng. 2023 Oct;51(10):2143-2171. doi: 10.1007/s10439-023-03321-y. Epub 2023 Jul 19. Ann Biomed Eng. 2023. PMID: 37468688 Review.
-
A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling.Stem Cells. 2011 Feb;29(2):367-75. doi: 10.1002/stem.580. Stem Cells. 2011. PMID: 21732493 Free PMC article.
-
Differentiation of vascular smooth muscle cells from local precursors during embryonic and adult arteriogenesis requires Notch signaling.Proc Natl Acad Sci U S A. 2012 May 1;109(18):6993-8. doi: 10.1073/pnas.1118512109. Epub 2012 Apr 16. Proc Natl Acad Sci U S A. 2012. PMID: 22509029 Free PMC article.
-
Generation of Endothelial Cells From Human Pluripotent Stem Cells.Arterioscler Thromb Vasc Biol. 2019 Jul;39(7):1317-1329. doi: 10.1161/ATVBAHA.119.312265. Epub 2019 May 9. Arterioscler Thromb Vasc Biol. 2019. PMID: 31242035 Free PMC article. Review.
References
-
- Kaufman DS, Lewis RL, Hanson ET, Auerbach R, Plendl J, Thomson JA. Functional endothelial cells derived from rhesus monkey embryonic stem cells. Blood. 2004;103(4):1325–1332. - PubMed
-
- Fennie C, Cheng J, Dowbenko D, Young P, Lasky LA. CD34+ endothelial cell lines derived from murine yolk sac induce the proliferation and differentiation of yolk sac CD34+ hematopoietic progenitors. Blood. 1995;86(12):4454–4467. - PubMed
-
- Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, Arzigian M, Fukumura D, Jain RK, Scadden DT. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25(3):317–318. - PubMed
-
- Mariappan D, Winkler J, Chen S, Schulz H, Hescheler J, Sachinidis A. Transcriptional profiling of CD31(+) cells isolated from murine embryonic stem cells. Genes Cells. 2009;14(2):243–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

