A mutation in the mouse Amelx tri-tyrosyl domain results in impaired secretion of amelogenin and phenocopies human X-linked amelogenesis imperfecta
- PMID: 20067920
- PMCID: PMC2838535
- DOI: 10.1093/hmg/ddq001
A mutation in the mouse Amelx tri-tyrosyl domain results in impaired secretion of amelogenin and phenocopies human X-linked amelogenesis imperfecta
Abstract
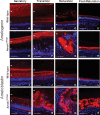
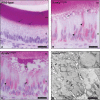
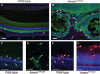
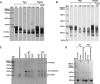
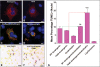
Amelogenesis imperfecta (AI) describes a broad group of clinically and genetically heterogeneous inherited defects of dental enamel bio-mineralization. Despite identification of a number of genetic mutations underlying AI, the precise causal mechanisms have yet to be determined. Using a multi-disciplinary approach, we describe here a mis-sense mutation in the mouse Amelx gene resulting in a Y --> H substitution in the tri-tyrosyl domain of the enamel extracellular matrix protein amelogenin. The enamel in affected animals phenocopies human X-linked AI where similar mutations have been reported. Animals affected by the mutation have severe defects of enamel bio-mineralization associated with absence of full-length amelogenin protein in the developing enamel matrix, loss of ameloblast phenotype, increased ameloblast apoptosis and formation of multi-cellular masses. We present evidence to demonstrate that affected ameloblasts express but fail to secrete full-length amelogenin leading to engorgement of the endoplasmic reticulum/Golgi apparatus. Immunohistochemical analysis revealed accumulations of both amelogenin and ameloblastin in affected cells. Co-transfection of Ambn and mutant Amelx in a eukaryotic cell line also revealed intracellular abnormalities and increased cytotoxicity compared with cells singly transfected with wild-type Amelx, mutant Amelx or Ambn or co-transfected with both wild-type Amelx and Ambn. We hypothesize that intracellular protein-protein interactions mediated via the amelogenin tri-tyrosyl motif are a key mechanistic factor underpinning the molecular pathogenesis in this example of AI. This study therefore successfully links phenotype with underlying genetic lesion in a relevant murine model for human AI.
Figures



References
-
- Backman B., Holm A.K. Amelogenesis imperfecta: prevalence and incidence in a northern Swedish county. CommunITY Dent. Oral Epidemiol. 1986;14:43–47. - PubMed
-
- Backman B. Amelogenesis imperfecta—clinical manifestations in 51 families in a northern Swedish county. Scand. J. Dent. Res. 1988;96:505–516. - PubMed
-
- Witkop C.J., Jr Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification. J. Oral Pathol. 1988;17:547–553. - PubMed
-
- Stephanopoulos G., Garefalaki M.E., Lyroudia K. Genes and related proteins involved in amelogenesis imperfecta. J. Dent. Res. 2005;84:1117–1126. - PubMed
-
- Hu J.C., Chun Y.H., Al Hazzazzi T., Simmer J.P. Enamel formation and amelogenesis imperfecta. Cells Tissues Organs. 2007;186:78–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

