Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new- onset type 1 diabetes
- PMID: 20067954
- PMCID: PMC2845036
- DOI: 10.2337/dc09-1349
Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new- onset type 1 diabetes
Abstract
Objective: This trial tested whether mycophenolate mofetil (MMF) alone or with daclizumab (DZB) could arrest the loss of insulin-producing beta-cells in subjects with new-onset type 1 diabetes.
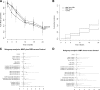
Research design and methods: A multi-center, randomized, placebo-controlled, double-masked trial was initiated by Type 1 Diabetes TrialNet at 13 sites in North America and Europe. Subjects diagnosed with type 1 diabetes and with sufficient C-peptide within 3 months of diagnosis were randomized to either MMF alone, MMF plus DZB, or placebo, and then followed for 2 years. The primary outcome was the geometric mean area under the curve (AUC) C-peptide from the 2-h mixed meal tolerance test.
Results: One hundred and twenty-six subjects were randomized and treated during the trial. The geometric mean C-peptide AUC at 2 years was unaffected by MMF alone or MMF plus DZB versus placebo. Adverse events were more frequent in the active therapy groups relative to the control group, but not significantly.
Conclusions: Neither MMF alone nor MMF in combination with DZB had an effect on the loss of C-peptide in subjects with new-onset type 1 diabetes. Higher doses or more targeted immunotherapies may be needed to affect the autoimmune process.
Figures

Comment in
-
Loss and preservation of beta-cell function: two treatment regimes targeting T or B lymphocytes.Curr Diab Rep. 2010 Oct;10(5):323-5. doi: 10.1007/s11892-010-0141-3. Curr Diab Rep. 2010. PMID: 20703833 No abstract available.
References
-
- Aly T, Devendra D, Eisenbarth GS. Immunotherapeutic approaches to prevent, ameliorate, and cure type 1 diabetes. Am J Ther 2005;12:481–490 - PubMed
-
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 - PubMed
-
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 - PubMed
-
- Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, Sayre P, Bianchine P, Wong E, Seyfert-Margolis V, Bourcier K, Bluestone JA. Immune Tolerance Network ITN007AI Study Group. Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol 2009;132:166–173 - PMC - PubMed
-
- DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

