Efficacy and safety of lacosamide in painful diabetic neuropathy
- PMID: 20067958
- PMCID: PMC2845038
- DOI: 10.2337/dc09-1578
Efficacy and safety of lacosamide in painful diabetic neuropathy
Abstract
Objective: To evaluate efficacy and safety of lacosamide compared with placebo in painful diabetic polyneuropathy.
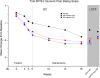
Research design and methods: Diabetic patients with at least moderate neuropathic pain were randomized to placebo or lacosamide 400 (in a slow or standard titration) or 600 mg/day over 6-week titration and 12-week maintenance periods. Primary efficacy criterion was intra-individual change in average daily Numeric Pain Rating Scale score from baseline to the last 4 weeks.
Results: For the primary end point, pain reduction was numerically but not statistically greater with lacosamide compared with placebo (400 mg/day, P = 0.12; 600 mg/day, P = 0.18). Both doses were significantly more effective compared with placebo over the titration (P = 0.03, P = 0.006), maintenance (P = 0.01, P = 0.005), and entire treatment periods (P = 0.03, P = 0.02). Safety profiles between titration schemes were similar.
Conclusions: Lacosamide reduced neuropathic pain and was well tolerated in diabetic patients, but the primary efficacy criterion was not met, possibly due to an increased placebo response over the last 4 weeks.
Figures
References
-
- Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518–1522 - PubMed
-
- Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. KORA Study Group. KORA Study Group. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009;10:393–400 - PubMed
-
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962 - PubMed
-
- Hovinga CA. SPM-927 (Schwarz Pharma). IDrugs 2003;6:479–485 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

