Functional characterization of protein-sorting machineries at the trans-Golgi network in Drosophila melanogaster
- PMID: 20067992
- PMCID: PMC2816189
- DOI: 10.1242/jcs.055103
Functional characterization of protein-sorting machineries at the trans-Golgi network in Drosophila melanogaster
Abstract
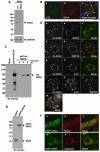
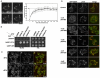
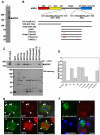
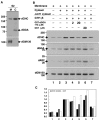
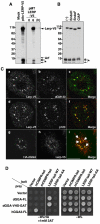
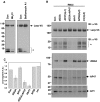
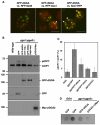
Targeting of proteins to their final destination is a prerequisite for living cells to maintain their homeostasis. Clathrin functions as a coat that forms transport carriers called clathrin-coated vesicles (CCVs) at the plasma membrane and post-Golgi compartments. In this study, we established an experimental system using Schneider S2 cells derived from the fruit fly, Drosophila melanogaster, as a model system to study the physiological roles of clathrin adaptors, and to dissect the processes of CCV formation. We found that a clathrin adaptor Drosophila GGA (dGGA), a homolog of mammalian GGA proteins, localizes to the trans-Golgi network (TGN) and is capable of recruiting clathrin from the cytosol onto TGN membranes. dGGA itself is recruited from the cytosol to the TGN in an ARF1 small GTPase (dARF79F)-dependent manner. dGGA recognizes the cytoplasmic acidic-cluster-dileucine (ACLL) sorting signal of Lerp (lysosomal enzyme receptor protein), a homolog of mammalian mannose 6-phosphate receptors. Moreover, both dGGA and another type of TGN-localized clathrin adaptor, AP-1 (adaptor protein-1 complex), are shown to be involved in the trafficking of Lerp from the TGN to endosomes and/or lysosomes. Taken together, our findings indicate that the protein-sorting machinery in fly cells is well conserved relative to that in mammals, enabling the use of fly cells to dissect CCV biogenesis and clathrin-dependent protein trafficking at the TGN of higher eukaryotes.
Figures

Similar articles
-
Protein complexes containing CYFIP/Sra/PIR121 coordinate Arf1 and Rac1 signalling during clathrin-AP-1-coated carrier biogenesis at the TGN.Nat Cell Biol. 2010 Apr;12(4):330-40. doi: 10.1038/ncb2034. Epub 2010 Mar 14. Nat Cell Biol. 2010. PMID: 20228810 Free PMC article.
-
Clathrin terminal domain-ligand interactions regulate sorting of mannose 6-phosphate receptors mediated by AP-1 and GGA adaptors.J Biol Chem. 2014 Feb 21;289(8):4906-18. doi: 10.1074/jbc.M113.535211. Epub 2014 Jan 9. J Biol Chem. 2014. PMID: 24407285 Free PMC article.
-
Phosphoinositide-mediated clathrin adaptor progression at the trans-Golgi network.Nat Cell Biol. 2012 Feb 19;14(3):239-48. doi: 10.1038/ncb2427. Nat Cell Biol. 2012. PMID: 22344030 Free PMC article.
-
Visualization of TGN-endosome trafficking in mammalian and Drosophila cells.Methods Enzymol. 2012;504:255-71. doi: 10.1016/B978-0-12-391857-4.00013-6. Methods Enzymol. 2012. PMID: 22264539 Review.
-
Receptor-mediated sorting of soluble vacuolar proteins: myths, facts, and a new model.J Exp Bot. 2016 Aug;67(15):4435-49. doi: 10.1093/jxb/erw222. Epub 2016 Jun 4. J Exp Bot. 2016. PMID: 27262127 Review.
Cited by
-
AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila.Mol Biol Cell. 2011 Jun 15;22(12):2094-105. doi: 10.1091/mbc.E11-01-0054. Epub 2011 Apr 13. Mol Biol Cell. 2011. PMID: 21490149 Free PMC article.
-
Drosophila melanogaster cellular repressor of E1A-stimulated genes is a lysosomal protein essential for fly development.Biochim Biophys Acta. 2014 Dec;1843(12):2900-12. doi: 10.1016/j.bbamcr.2014.08.012. Epub 2014 Aug 27. Biochim Biophys Acta. 2014. PMID: 25173815 Free PMC article.
-
Type II phosphatidylinositol 4-kinase regulates trafficking of secretory granule proteins in Drosophila.Development. 2012 Aug;139(16):3040-50. doi: 10.1242/dev.077644. Epub 2012 Jul 12. Development. 2012. PMID: 22791894 Free PMC article.
-
Induction of membrane curvature by proteins involved in Golgi trafficking.Adv Biol Regul. 2020 Jan;75:100661. doi: 10.1016/j.jbior.2019.100661. Epub 2019 Oct 16. Adv Biol Regul. 2020. PMID: 31668661 Free PMC article. Review.
-
Drosophila GGA model: an ultimate gateway to GGA analysis.Traffic. 2011 Dec;12(12):1821-38. doi: 10.1111/j.1600-0854.2011.01285.x. Epub 2011 Oct 13. Traffic. 2011. PMID: 21923734 Free PMC article.
References
-
- Bai H., Doray B., Kornfeld S. (2004). GGA1 interacts with the adaptor protein AP-1 through a WNSF sequence in its hinge region. J. Biol. Chem. 279, 17411-17417 - PubMed
-
- Bonifacino J. S. (2004). The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell. Biol. 5, 23-32 - PubMed
-
- Bonifacino J. S., Lippincott-Schwartz J. (2003). Coat proteins: shaping membrane transport. Nat. Rev. Mol. Cell. Biol. 4, 409-414 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

