Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: a three-dimensional FRET study
- PMID: 20067998
- PMCID: PMC2816188
- DOI: 10.1242/jcs.053793
Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: a three-dimensional FRET study
Abstract
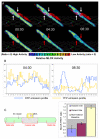
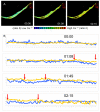
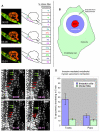
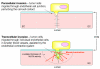
The transient and localized signaling events between invasive breast cancer cells and the underlying endothelial cells have remained poorly characterized. We report a novel approach integrating vascular engineering with three-dimensional time-lapse fluorescence resonance energy transfer (FRET) imaging to dissect how endothelial myosin light chain kinase (MLCK) is modulated during tumor intravasation. We show that tumor transendothelial migration occurs via both paracellular (i.e. through cell-cell junctions) and transcellular (i.e. through individual endothelial cells) routes. Endothelial MLCK is activated at the invasion site, leading to regional diphosphorylation of myosin-II regulatory light chain (RLC) and myosin contraction. Blocking endothelial RLC diphosphorylation blunts tumor transcellular, but not paracellular, invasion. Our results implicate an important role for endothelial myosin-II function in tumor intravasation.
Figures




Similar articles
-
Structure and biomechanics of the endothelial transcellular circumferential invasion array in tumor invasion.PLoS One. 2014 Feb 24;9(2):e89758. doi: 10.1371/journal.pone.0089758. eCollection 2014. PLoS One. 2014. PMID: 24587014 Free PMC article.
-
Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2.J Biol Chem. 2000 Jun 16;275(24):18366-74. doi: 10.1074/jbc.M001339200. J Biol Chem. 2000. PMID: 10748018
-
MLCK and ROCK mutualism in endothelial barrier dysfunction.Biochimie. 2020 Jan;168:83-91. doi: 10.1016/j.biochi.2019.10.010. Epub 2019 Oct 24. Biochimie. 2020. PMID: 31668993
-
Myosin light chain kinase in microvascular endothelial barrier function.Cardiovasc Res. 2010 Jul 15;87(2):272-80. doi: 10.1093/cvr/cvq144. Epub 2010 May 17. Cardiovasc Res. 2010. PMID: 20479130 Free PMC article. Review.
-
Phosphorylation of myosin regulatory light chain by myosin light chain kinase, and muscle contraction.Circ J. 2009 Feb;73(2):208-13. doi: 10.1253/circj.cj-08-1041. Epub 2008 Dec 26. Circ J. 2009. PMID: 19110504 Review.
Cited by
-
Reduced Lamin A/C Does Not Facilitate Cancer Cell Transendothelial Migration but Compromises Lung Metastasis.Cancers (Basel). 2021 May 14;13(10):2383. doi: 10.3390/cancers13102383. Cancers (Basel). 2021. PMID: 34069191 Free PMC article.
-
The Heterogeneity of Neutrophil Recruitment in the Tumor Microenvironment and the Formation of Premetastatic Niches.J Immunol Res. 2021 Feb 19;2021:6687474. doi: 10.1155/2021/6687474. eCollection 2021. J Immunol Res. 2021. PMID: 33688508 Free PMC article. Review.
-
Novel three dimensional human endocervix cultures respond to 28-day hormone treatment.Endocrinology. 2015 Apr;156(4):1602-9. doi: 10.1210/en.2014-1840. Epub 2015 Jan 30. Endocrinology. 2015. PMID: 25635622 Free PMC article.
-
Pathway-based classification of cancer subtypes.Biol Direct. 2012 Jul 3;7:21. doi: 10.1186/1745-6150-7-21. Biol Direct. 2012. PMID: 22759382 Free PMC article.
-
Inside-out regulation of ICAM-1 dynamics in TNF-alpha-activated endothelium.PLoS One. 2010 Jun 28;5(6):e11336. doi: 10.1371/journal.pone.0011336. PLoS One. 2010. PMID: 20596527 Free PMC article.
References
-
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 2026-2029 - PubMed
-
- Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibuchi K. (1997). Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275, 1308-1311 - PubMed
-
- Bell S. E., Mavila A., Salazar R., Bayless K. J., Kanagala S., Maxwell S. A., Davis G. E. (2001). Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J. Cell Sci. 114, 2755-2773 - PubMed
-
- Bresnick A. R. (1999). Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 11, 26-33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

