Lupus-prone New Zealand Black/New Zealand White F1 mice display endothelial dysfunction and abnormal phenotype and function of endothelial progenitor cells
- PMID: 20068018
- PMCID: PMC3151666
- DOI: 10.1177/0961203309353773
Lupus-prone New Zealand Black/New Zealand White F1 mice display endothelial dysfunction and abnormal phenotype and function of endothelial progenitor cells
Abstract
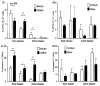
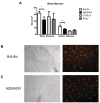
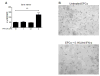
Patients with systemic lupus erythematosus (SLE) have an impairment in phenotype and function of endothelial progenitor cells (EPCs) which is mediated by interferon alpha (IFN-alpha). We assessed whether murine lupus models also exhibit vasculogenesis abnormalities and their potential association with endothelial dysfunction. Phenotype and function of EPCs and type I IFN gene signatures in EPC compartments were assessed in female New Zealand Black/New Zealand White F(1) (NZB/W), B6.MRL-Fas(lpr)/J (B6/lpr) and control mice. Thoracic aorta endothelial and smooth muscle function were measured in response to acetylcholine or sodium nitropruside, respectively. NZB/W mice displayed reduced numbers, increased apoptosis and impaired function of EPCs. These abnormalities correlated with significant decreases in endothelium-dependent vasomotor responses and with increased type I IFN signatures in EPC compartments. In contrast, B6/lpr mice showed improvement in endothelium-dependent and endothelial-independent responses, no abnormalities in EPC phenotype or function and downregulation of type I IFN signatures in EPC compartments. These results indicate that NZB/W mice represent a good model to study the mechanisms leading to endothelial dysfunction and abnormal vasculogenesis in lupus. These results further support the hypothesis that type I IFNs may play an important role in premature vascular damage and, potentially, atherosclerosis development in SLE.
Conflict of interest statement
The author(s) declare that they have no competing interests
Figures


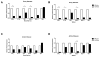

References
-
- Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997 Mar 1;145(5):408–15. - PubMed
-
- Aranow C, Ginzler EM. Epidemiology of cardiovascular disease in systemic lupus erythematosus. Lupus. 2000;9(3):166–9. - PubMed
-
- Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001 Oct;44(10):2331–7. - PubMed
-
- Rajagopalan S, Somers EC, Brook RD, Kehrer C, Pfenninger D, Lewis E, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004 May 15;103(10):3677–83. - PubMed
-
- Roman MJ, Devereux RB, Schwartz JE, Lockshin MD, Paget SA, Davis A, et al. Arterial stiffness in chronic inflammatory diseases. Hypertension. 2005 Jul;46(1):194–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

