Engineering anti-vascular endothelial growth factor single chain disulfide-stabilized antibody variable fragments (sc-dsFv) with phage-displayed sc-dsFv libraries
- PMID: 20068035
- PMCID: PMC2832938
- DOI: 10.1074/jbc.M109.061457
Engineering anti-vascular endothelial growth factor single chain disulfide-stabilized antibody variable fragments (sc-dsFv) with phage-displayed sc-dsFv libraries
Abstract
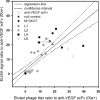
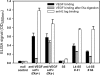
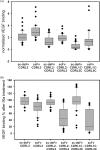
Phage display of antibody fragments from natural or synthetic antibody libraries with the single chain constructs combining the variable fragments (scFv) has been one of the most prominent technologies in antibody engineering. However, the nature of the artificial single chain constructs results in unstable proteins expressed on the phage surface or as soluble proteins secreted in the bacterial culture medium. The stability of the variable domain structures can be enhanced with interdomain disulfide bond, but the single chain disulfide-stabilized constructs (sc-dsFv) have yet to be established as a feasible format for bacterial phage display due to diminishing expression levels on the phage surface in known phage display systems. In this work, biological combinatorial searches were used to establish that the c-region of the signal sequence is critically responsible for effective expression and functional folding of the sc-dsFv on the phage surface. The optimum signal sequences increase the expression of functional sc-dsFv by 2 orders of magnitude compared with wild-type signal sequences, enabling the construction of phage-displayed synthetic antivascular endothelial growth factor sc-dsFv libraries. Comparison of the scFv and sc-dsFv variants selected from the phage-displayed libraries for vascular endothelial growth factor binding revealed the sequence preference differences resulting from the interdomain disulfide bond. These results underlie a new phage display format for antibody fragments with all the benefits from the scFv format but without the downside due to the instability of the dimeric interface in scFv.
Figures



Similar articles
-
Signal sequence as a determinant in expressing disulfide-stabilized single chain antibody variable fragments (sc-dsFv) against human VEGF.Mol Biosyst. 2010 Jul;6(7):1307-15. doi: 10.1039/b921106c. Epub 2010 Apr 27. Mol Biosyst. 2010. PMID: 20424732
-
Effects of signal sequence on phage-displayed disulfide-stabilized single chain antibody variable fragment (sc-dsFv) libraries.Biochem Biophys Res Commun. 2011 Jul 29;411(2):348-53. doi: 10.1016/j.bbrc.2011.06.146. Epub 2011 Jun 28. Biochem Biophys Res Commun. 2011. PMID: 21741355
-
Phage display of disulfide-stabilized Fv fragments.J Immunol Methods. 1995 May 11;182(1):41-50. doi: 10.1016/0022-1759(95)00016-4. J Immunol Methods. 1995. PMID: 7769243
-
Antibody engineering of recombinant Fv immunotoxins for improved targeting of cancer: disulfide-stabilized Fv immunotoxins.Clin Cancer Res. 1996 Feb;2(2):245-52. Clin Cancer Res. 1996. PMID: 9816166 Review.
-
Engineering antibody Fv fragments for cancer detection and therapy: disulfide-stabilized Fv fragments.Nat Biotechnol. 1996 Oct;14(10):1239-45. doi: 10.1038/nbt1096-1239. Nat Biotechnol. 1996. PMID: 9631086 Review.
Cited by
-
Toward Drug-Like Multispecific Antibodies by Design.Int J Mol Sci. 2020 Oct 12;21(20):7496. doi: 10.3390/ijms21207496. Int J Mol Sci. 2020. PMID: 33053650 Free PMC article. Review.
-
Antibody phage display libraries: contributions to oncology.Int J Mol Sci. 2012;13(5):5420-5440. doi: 10.3390/ijms13055420. Epub 2012 May 4. Int J Mol Sci. 2012. PMID: 22754305 Free PMC article. Review.
-
Identification of two aberrant transcripts derived from a hybridoma with amplification of functional immunoglobulin variable genes.Cell Mol Immunol. 2010 Sep;7(5):349-54. doi: 10.1038/cmi.2010.33. Epub 2010 Jul 26. Cell Mol Immunol. 2010. PMID: 20657605 Free PMC article.
-
Rationalization and design of the complementarity determining region sequences in an antibody-antigen recognition interface.PLoS One. 2012;7(3):e33340. doi: 10.1371/journal.pone.0033340. Epub 2012 Mar 22. PLoS One. 2012. PMID: 22457753 Free PMC article.
-
Expression, production, and renaturation of a functional single-chain variable antibody fragment (scFv) against human ICAM-1.Braz J Med Biol Res. 2014 Jul;47(7):540-7. doi: 10.1590/1414-431x20143276. Epub 2014 Jun 4. Braz J Med Biol Res. 2014. PMID: 24919171 Free PMC article.
References
-
- Yokota T., Milenic D. E., Whitlow M., Schlom J. (1992) Cancer Res. 52, 3402–3408 - PubMed
-
- Michnick S. W., Sidhu S. S. (2008) Nat. Chem. Biol. 4, 326–329 - PubMed
-
- Wark K. L., Hudson P. J. (2006) Adv. Drug Deliv. Rev. 58, 657–670 - PubMed
-
- Carter P. J. (2006) Nat. Rev. Immunol. 6, 343–357 - PubMed
-
- Hoogenboom H. R. (2005) Nat. Biotechnol. 23, 1105–1116 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

