A novel mechanism of P-type ATPase autoinhibition involving both termini of the protein
- PMID: 20068040
- PMCID: PMC2844182
- DOI: 10.1074/jbc.M109.096123
A novel mechanism of P-type ATPase autoinhibition involving both termini of the protein
Abstract
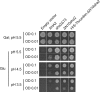
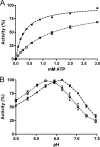
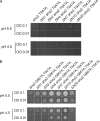
The activity of many P-type ATPases is found to be regulated by interacting proteins or autoinhibitory elements located in N- or C-terminal extensions. An extended C terminus of fungal and plant P-type plasma membrane H(+)-ATPases has long been recognized to be part of a regulatory apparatus involving an autoinhibitory domain. Here we demonstrate that both the N and the C termini of the plant plasma membrane H(+)-ATPase are directly involved in controlling the pump activity state and that N-terminal displacements are coupled to secondary modifications taking place at the C-terminal end. This identifies the first group of P-type ATPases for which both ends of the polypeptide chain constitute regulatory domains, which together contribute to the autoinhibitory apparatus. This suggests an intricate mechanism of cis-regulation with both termini of the protein communicating to obtain the necessary control of the enzyme activity state.
Figures




Similar articles
-
Specific Activation of the Plant P-type Plasma Membrane H+-ATPase by Lysophospholipids Depends on the Autoinhibitory N- and C-terminal Domains.J Biol Chem. 2015 Jun 26;290(26):16281-91. doi: 10.1074/jbc.M114.617746. Epub 2015 May 13. J Biol Chem. 2015. PMID: 25971968 Free PMC article.
-
Molecular dissection of the C-terminal regulatory domain of the plant plasma membrane H+-ATPase AHA2: mapping of residues that when altered give rise to an activated enzyme.Biochemistry. 1999 Jun 1;38(22):7227-34. doi: 10.1021/bi982482l. Biochemistry. 1999. PMID: 10353834
-
Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+ -ATPase by preventing interaction with 14-3-3 protein.Plant Cell. 2007 May;19(5):1617-34. doi: 10.1105/tpc.105.035626. Epub 2007 May 4. Plant Cell. 2007. PMID: 17483306 Free PMC article.
-
Regulation of the plasma membrane proton pump (H(+)-ATPase) by phosphorylation.Curr Opin Plant Biol. 2015 Dec;28:68-75. doi: 10.1016/j.pbi.2015.09.005. Epub 2015 Oct 24. Curr Opin Plant Biol. 2015. PMID: 26476298 Free PMC article. Review.
-
The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles.Pflugers Arch. 2009 Jan;457(3):645-55. doi: 10.1007/s00424-008-0457-x. Epub 2008 Jan 29. Pflugers Arch. 2009. PMID: 18228034 Review.
Cited by
-
General and specific interactions of the phospholipid bilayer with P-type ATPases.Biophys Rev. 2019 Jun;11(3):353-364. doi: 10.1007/s12551-019-00533-2. Epub 2019 May 9. Biophys Rev. 2019. PMID: 31073955 Free PMC article. Review.
-
P-type transport ATPases in Leishmania and Trypanosoma.Parasite. 2019;26:69. doi: 10.1051/parasite/2019069. Epub 2019 Nov 29. Parasite. 2019. PMID: 31782726 Free PMC article. Review.
-
Low ABA concentration promotes root growth and hydrotropism through relief of ABA INSENSITIVE 1-mediated inhibition of plasma membrane H+-ATPase 2.Sci Adv. 2021 Mar 17;7(12):eabd4113. doi: 10.1126/sciadv.abd4113. Print 2021 Mar. Sci Adv. 2021. PMID: 33731345 Free PMC article.
-
Autoinhibitory regulation of TrwK, an essential VirB4 ATPase in type IV secretion systems.J Biol Chem. 2011 May 13;286(19):17376-82. doi: 10.1074/jbc.M110.208942. Epub 2011 Mar 24. J Biol Chem. 2011. PMID: 21454654 Free PMC article.
-
Autoinhibition and regulation by phosphoinositides of ATP8B1, a human lipid flippase associated with intrahepatic cholestatic disorders.Elife. 2022 Apr 13;11:e75272. doi: 10.7554/eLife.75272. Elife. 2022. PMID: 35416773 Free PMC article.
References
-
- Toyoshima C., Nakasako M., Nomura H., Ogawa H. (2000) Nature 405, 647–655 - PubMed
-
- Morth J. P., Pedersen B. P., Toustrup-Jensen M. S., Sørensen T. L., Petersen J., Andersen J. P., Vilsen B., Nissen P. (2007) Nature 450, 1043–1049 - PubMed
-
- Pedersen B. P., Buch-Pedersen M. J., Morth J. P., Palmgren M. G., Nissen P. (2007) Nature 450, 1111–1114 - PubMed
-
- Pufall M. A., Graves B. J. (2002) Annu. Rev. Cell Dev. Biol. 18, 421–462 - PubMed
-
- Palmgren M. G., Sommarin M., Serrano R., Larsson C. (1991) J. Biol. Chem. 266, 20470–20475 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

