Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts
- PMID: 20068043
- PMCID: PMC2832969
- DOI: 10.1074/jbc.M109.031575
Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts
Abstract
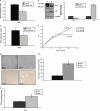
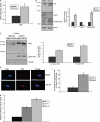
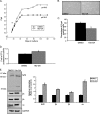
Replicative senescence in human fibroblasts is accompanied with alterations of various biological processes, including the impaired function of the proteasome. The proteasome is responsible for the removal of both normal and damaged proteins. Due to its latter function, proteasome is also considered a representative secondary antioxidant cellular mechanism. Nrf2 is a basic transcription factor responsible for the regulation of the cellular antioxidant response that has also been shown to regulate several proteasome subunits in mice. We have established in this study the proteasome-related function of Nrf2 in human fibroblasts undergoing replicative senescence. We demonstrate that Nrf2 has a declined function in senescence, whereas its silencing leads to premature senescence. However, upon its activation by a novel Nrf2 inducer, elevated levels of proteasome activity and content are recorded only in cell lines possessing a functional Nrf2. Moreover, treatment by the Nrf2 inducer results in the enhanced survival of cells following oxidative stress, whereas continuous treatment leads to lifespan extension of human fibroblasts. Importantly the Nrf2-proteasome axis is functional in terminally senescent cultures as these cells retain their responsiveness to the Nrf2 stimuli. In conclusion, these findings open up new directions for future manipulation of the senescence phenotype.
Figures




Similar articles
-
Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence.Mol Biol Cell. 2013 Jun;24(12):1852-62. doi: 10.1091/mbc.E12-09-0666. Epub 2013 May 1. Mol Biol Cell. 2013. PMID: 23637463 Free PMC article.
-
The olive constituent oleuropein exhibits proteasome stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts.Rejuvenation Res. 2007 Jun;10(2):157-72. doi: 10.1089/rej.2006.0513. Rejuvenation Res. 2007. PMID: 17518699
-
Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation.J Biol Chem. 2003 Jul 25;278(30):28026-37. doi: 10.1074/jbc.M301048200. Epub 2003 May 7. J Biol Chem. 2003. PMID: 12736271
-
Targeting the KEAP1-NRF2 System to Prevent Kidney Disease Progression.Am J Nephrol. 2017;45(6):473-483. doi: 10.1159/000475890. Epub 2017 May 13. Am J Nephrol. 2017. PMID: 28502971 Review.
-
Nrf2 in early vascular ageing: Calcification, senescence and therapy.Clin Chim Acta. 2020 Jun;505:108-118. doi: 10.1016/j.cca.2020.02.026. Epub 2020 Feb 22. Clin Chim Acta. 2020. PMID: 32097628 Review.
Cited by
-
The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging.Oxid Med Cell Longev. 2021 Apr 19;2021:6635460. doi: 10.1155/2021/6635460. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34012501 Free PMC article. Review.
-
The Role of Nrf2 in the Antioxidant Cellular Response to Medical Ozone Exposure.Int J Mol Sci. 2019 Aug 17;20(16):4009. doi: 10.3390/ijms20164009. Int J Mol Sci. 2019. PMID: 31426459 Free PMC article. Review.
-
Advances in Proteasome Enhancement by Small Molecules.Biomolecules. 2021 Nov 30;11(12):1789. doi: 10.3390/biom11121789. Biomolecules. 2021. PMID: 34944433 Free PMC article. Review.
-
Modulation of the ubiquitin-proteasome system by marine natural products.Redox Biol. 2021 May;41:101897. doi: 10.1016/j.redox.2021.101897. Epub 2021 Feb 17. Redox Biol. 2021. PMID: 33640701 Free PMC article. Review.
-
Sodium Sulfide Attenuates Ischemic-Induced Heart Failure by Enhancing Proteasomal Function in an Nrf2-Dependent Manner.Circ Heart Fail. 2016 Apr;9(4):e002368. doi: 10.1161/CIRCHEARTFAILURE.115.002368. Circ Heart Fail. 2016. PMID: 27056879 Free PMC article.
References
-
- Petropoulou C., Chondrogianni N., Simões D., Agiostratidou G., Drosopoulos N., Kotsota V., Gonos E. S. (2000) Ann. N.Y. Acad. Sci. 908, 133–142 - PubMed
-
- Collado M., Blasco M. A., Serrano M. (2007) Cell 130, 223–233 - PubMed
-
- Chondrogianni N., Gonos E. S. (2005) Exp. Gerontol. 40, 931–938 - PubMed
-
- Chondrogianni N., Stratford F. L., Trougakos I. P., Friguet B., Rivett A. J., Gonos E. S. (2003) J. Biol. Chem. 278, 28026–28037 - PubMed
-
- Chondrogianni N., Gonos E. S. (2004) Biogerontology 5, 55–61 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

