Alkaline ceramidase 3 (ACER3) hydrolyzes unsaturated long-chain ceramides, and its down-regulation inhibits both cell proliferation and apoptosis
- PMID: 20068046
- PMCID: PMC2832947
- DOI: 10.1074/jbc.M109.063586
Alkaline ceramidase 3 (ACER3) hydrolyzes unsaturated long-chain ceramides, and its down-regulation inhibits both cell proliferation and apoptosis
Abstract
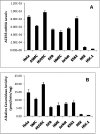
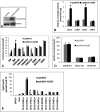
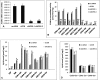
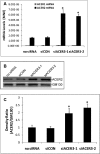
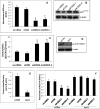
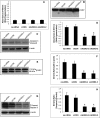
Ceramides with different fatty acyl chains may vary in their physiological or pathological roles; however, it remains unclear how cellular levels of individual ceramide species are regulated. Here, we demonstrate that our previously cloned human alkaline ceramidase 3 (ACER3) specifically controls the hydrolysis of ceramides carrying unsaturated long acyl chains, unsaturated long-chain (ULC) ceramides. In vitro, ACER3 only hydrolyzed C(18:1)-, C(20:1)-, C(20:4)-ceramides, dihydroceramides, and phytoceramides. In cells, ACER3 overexpression decreased C(18:1)- and C(20:1)-ceramides and dihydroceramides, whereas ACER3 knockdown by RNA interference had the opposite effect, suggesting that ACER3 controls the catabolism of ULC ceramides and dihydroceramides. ACER3 knockdown inhibited cell proliferation and up-regulated the cyclin-dependent kinase inhibitor p21(CIP1/WAF1). Blocking p21(CIP1/WAF1) up-regulation attenuated the inhibitory effect of ACER3 knockdown on cell proliferation, suggesting that ACER3 knockdown inhibits cell proliferation because of p21(CIP1/WAF1) up-regulation. ACER3 knockdown inhibited cell apoptosis in response to serum deprivation. ACER3 knockdown up-regulated the expression of the alkaline ceramidase 2 (ACER2), and the ACER2 up-regulation decreased non-ULC ceramide species while increasing both sphingosine and its phosphate. Collectively, these data suggest that ACER3 catalyzes the hydrolysis of ULC ceramides and dihydroceramides and that ACER3 coordinates with ACER2 to regulate cell proliferation and survival.
Figures


References
-
- Charles A. G., Han T. Y., Liu Y. Y., Hansen N., Giuliano A. E., Cabot M. C. (2001) Cancer Chemother. Pharmacol. 47, 444–450 - PubMed
-
- Magnoni C., Euclidi E., Benassi L., Bertazzoni G., Cossarizza A., Seidenari S., Giannetti A. (2002) Toxicol. In Vitro 16, 349–355 - PubMed
-
- Jaffrézou J. P., Bruno A. P., Moisand A., Levade T., Laurent G. (2001) FASEB J. 15, 123–133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

