HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors
- PMID: 20068073
- PMCID: PMC3682507
- DOI: 10.1158/1078-0432.CCR-09-1322
HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors
Abstract
Purpose: Glioblastoma multiforme (GBM) is the most aggressive human primary brain tumor and is currently incurable. Immunotherapies have the potential to target GBM stem cells, which are resistant to conventional therapies. Human epidermal growth factor receptor 2 (HER2) is a validated immunotherapy target, and we determined if HER2-specific T cells can be generated from GBM patients that will target autologous HER2-positive GBMs and their CD133-positive stem cell compartment.
Experimental design: HER2-specific T cells from 10 consecutive GBM patients were generated by transduction with a retroviral vector encoding a HER2-specific chimeric antigen receptor. The effector function of HER2-specific T cells against autologous GBM cells, including CD133-positive stem cells, was evaluated in vitro and in an orthotopic murine xenograft model.
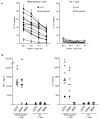
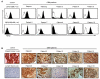
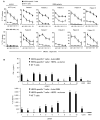
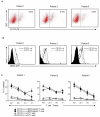
Results: Stimulation of HER2-specific T cells with HER2-positive autologous GBM cells resulted in T-cell proliferation and secretion of IFN-gamma and interleukin-2 in a HER2-dependent manner. Patients' HER2-specific T cells killed CD133-positive and CD133-negative cells derived from primary HER2-positive GBMs, whereas HER2-negative tumor cells were not killed. Injection of HER2-specific T cells induced sustained regression of autologous GBM xenografts established in the brain of severe combined immunodeficient mice.
Conclusions: Gene transfer allows the reliable generation of HER2-specific T cells from GBM patients, which have potent antitumor activity against autologous HER2-positive tumors including their putative stem cells. Hence, the adoptive transfer of HER2-redirected T cells may be a promising immunotherapeutic approach for GBM.
Figures




References
-
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. - PubMed
-
- Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30:10–14. - PubMed
-
- Stupp R, Mason WP, Van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Altaner C. Glioblastoma and stem cells. Neoplasma. 2008;55:369–374. - PubMed
-
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

