Secretory leukocyte protease inhibitor antagonizes paclitaxel in ovarian cancer cells
- PMID: 20068074
- PMCID: PMC2808000
- DOI: 10.1158/1078-0432.CCR-09-1979
Secretory leukocyte protease inhibitor antagonizes paclitaxel in ovarian cancer cells
Abstract
Purpose: Ovarian cancer recurrence with the development of paclitaxel resistance is an obstacle to long-term survival. We showed that secretory leukocyte protease inhibitor (SLPI) is a survival factor for ovarian cancer. We hypothesize that SLPI may antagonize paclitaxel injury.
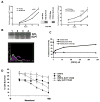
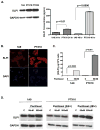
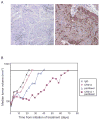
Experimental design: Differential SLPI induction in response to paclitaxel and in response to stable forced expression of SLPI was shown in A2780-1A9 cells and their paclitaxel-resistant sublines, PTX10 and PTX22, and confirmed with HEY-A8 cells. SLPI-mediated survival was reduced by the MAP/extracellular signal-regulated kinase (ERK) kinase inhibitor, U0126, and a humanized neutralizing monoclonal anti-SLPI antibody, CR012. OVCAR3 xenographs tested the role of CR012 in vivo.
Results: SLPI expression was lower in A2780-1A9 ovarian cancer cells than in PTX10 and PTX22, and SLPI was induced by paclitaxel exposure. Stable SLPI expression yielded a proliferation advantage (P = 0.01); expression of and response to SLPI in OVCAR3 cells were abrogated by exposure to CR012. SLPI reduced the paclitaxel susceptibility of 1A9 and HEY-A8 cells (P <or= 0.05), and SLPI expression did not increase the resistance of PTX10 and PTX22 cells. Both paclitaxel and SLPI overexpression induced ERK activation. Inhibition of MAP/ERK kinase with U0126 increased paclitaxel injury and overcame SLPI-mediated cell protection. It did not reinstate PTX10 sensitivity to paclitaxel, which was associated with AKT activation. Significant inhibition of OVCAR3 xenograft growth was observed with CR012 and paclitaxel, over single agents (P <or= 0.001).
Conclusions: A two-pronged approach confirmed that SLPI overcomes paclitaxel in part through activation of ERK1/2. These results credential SLPI as a molecular target for ovarian cancer and suggest CR012 as a tool for proof of concept.
Figures



References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA: a cancer journal for clinicians. 2008;58:71–96. - PubMed
-
- Einzig AI, Wiernik PH, Sasloff J, Runowicz CD, Goldberg GL. Phase II study and long-term follow-up of patients treated with taxol for advanced ovarian adenocarcinoma. J Clin Oncol. 1992;10:1748–53. - PubMed
-
- du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–9. - PubMed
-
- Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–16. - PubMed
-
- Hough CD, Cho KR, Zonderman AB, Schwartz DR, Morin PJ. Coordinately up-regulated genes in ovarian cancer. Cancer Res. 2001;61:3869–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

