Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2
- PMID: 20068097
- PMCID: PMC2943831
- DOI: 10.1158/1078-0432.CCR-09-2308
Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2
Abstract
Purpose: To evaluate clinical activity and target modulation of vandetanib in women with recurrent ovarian cancer.
Experimental design: A phase II trial of orally administered vandetanib 300 mg daily was designed to include analyses of target inhibition through paired biopsies and dynamic imaging. Core 18-gauge needle biopsies and dynamic contrast-enhanced magnetic resonance imaging were obtained before initiation of therapy and 6 weeks into therapy. Biopsy samples were subjected to reverse-phase protein lysate array endpoint analysis. Cytokine concentrations were measured by enzyme-linked immunosorbent assay in serially collected plasma samples.
Results: Twelve patients entered the study, and accrual was terminated in the first stage because of lack of response or disease stabilization beyond 6 months. Adverse events included rash, diarrhea, and prolonged QT interval corrected for heart rate, but not hypertension. Exploratory analyses showed that epidermal growth factor receptor (EGFR) phosphorylation was reduced in the eight paired biopsy sets obtained; vascular endothelial growth factor (VEGF) receptor-2 phosphorylation was not consistently affected nor were dynamic contrast-enhanced MRI permeability and flow parameters. Serial plasma VEGF concentrations were variable and did not significantly change in the 11 patients assessed.
Conclusions: Vandetanib 300 mg daily monotherapy had no significant clinical benefit in this disease setting. Proteomic analysis of paired biopsies detected both phosphorylated-EGFR and phosphorylated-VEGF receptor-2 in ovarian tumor tissue, but only phosphorylated-EGFR was measurably inhibited by vandetanib.
Figures


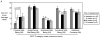
References
-
- Fleming GF, Ronnett BM, Seidman J, Zaino RJ, Rubin SC. Epithelian Ovarian Cancer. In: Barakat RR, Markman M, Randall ME, editors. Principles and Practices of Gynecologic Oncology. 5th ed Lippincott, Williams and Wilkins; Baltimore: 2009. pp. 763–836.
-
- Palayekar MJ, Herzog TJ. The emerging role of epidermal growth factor receptor inhibitors in ovarian cancer. Int J Gynecol Cancer. 2008;18:879–90. - PubMed
-
- Annunziata CM, Azad NS, Hoskins ER. E.C. K. Tumor invasion, angiogenesis and metastasis: biology and clinical application. In: Barakat RR, Markman M, Randall ME, editors. Principles and Practices of Gynecologic Oncology. 5th ed Lippincott, Williams and Wilkins; Baltimore: 2009. pp. 71–84.
-
- Martin L, Schilder R. Novel approaches in advancing the treatment of epithelial ovarian cancer: the role of angiogenesis inhibition. J Clin Oncol. 2007;25:2894–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

