Activity of the kinesin spindle protein inhibitor ispinesib (SB-715992) in models of breast cancer
- PMID: 20068098
- PMCID: PMC2844774
- DOI: 10.1158/1078-0432.CCR-09-1498
Activity of the kinesin spindle protein inhibitor ispinesib (SB-715992) in models of breast cancer
Abstract
Purpose: Ispinesib (SB-715992) is a potent inhibitor of kinesin spindle protein, a kinesin motor protein essential for the formation of a bipolar mitotic spindle and cell cycle progression through mitosis. Clinical studies of ispinesib have shown a 9% response rate in patients with locally advanced or metastatic breast cancer and a favorable safety profile without significant neurotoxicities, gastrointestinal toxicities, or hair loss. To better understand the potential of ispinesib in the treatment of breast cancer, we explored the activity of ispinesib alone and in combination with several therapies approved for the treatment of breast cancer.
Experimental design: We measured the ispinesib sensitivity and pharmacodynamic response of breast cancer cell lines representative of various subtypes in vitro and as xenografts in vivo and tested the ability of ispinesib to enhance the antitumor activity of approved therapies.
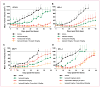
Results: In vitro, ispinesib displayed broad antiproliferative activity against a panel of 53 breast cell lines. In vivo, ispinesib produced regressions in each of five breast cancer models and tumor-free survivors in three of these models. The effects of ispinesib treatment on pharmacodynamic markers of mitosis and apoptosis were examined in vitro and in vivo, revealing a greater increase in both mitotic and apoptotic markers in the MDA-MB-468 model than in the less sensitive BT-474 model. In vivo, ispinesib enhanced the antitumor activity of trastuzumab, lapatinib, doxorubicin, and capecitabine and exhibited activity comparable with paclitaxel and ixabepilone.
Conclusions: These findings support further clinical exploration of kinesin spindle protein inhibitors for the treatment of breast cancer.
Conflict of interest statement
J.W. Purcell, J. Davis, M. Reddy, S. Martin, K. Samoya, H. Vo, K. Thomsen, P. Bean, K.W. Wood, and S. Cases are employees and consultants and/or have an ownership interest in Cytokinetics. J.W. Gray is a consultant for Agendia, Cepheid, Sirna/Merck, New Leaf Ventures, and Aveo Pharmaceuticals and received grant support from Cytokinetics, Inc. The other authors disclosed no potential conflicts of interest.
Figures



Similar articles
-
Initial testing (stage 1) of the kinesin spindle protein inhibitor ispinesib by the pediatric preclinical testing program.Pediatr Blood Cancer. 2009 Dec 15;53(7):1255-63. doi: 10.1002/pbc.22056. Pediatr Blood Cancer. 2009. PMID: 19554570 Free PMC article.
-
Inhibitor library screening identifies ispinesib as a new potential chemotherapeutic agent for pancreatic cancers.Cancer Sci. 2021 Nov;112(11):4641-4654. doi: 10.1111/cas.15134. Epub 2021 Oct 2. Cancer Sci. 2021. PMID: 34510663 Free PMC article.
-
Optimized S-trityl-L-cysteine-based inhibitors of kinesin spindle protein with potent in vivo antitumor activity in lung cancer xenograft models.J Med Chem. 2013 Mar 14;56(5):1878-93. doi: 10.1021/jm3014597. Epub 2013 Feb 27. J Med Chem. 2013. PMID: 23394180 Free PMC article.
-
[Lapatinib treatment-option in trastuzumab-resistant breast cancer].Magy Onkol. 2009 Dec;53(4):369-75. doi: 10.1556/MOnkol.53.2009.4.6. Magy Onkol. 2009. PMID: 20071309 Review. Hungarian.
-
Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases.Clin Ther. 2008 Aug;30(8):1426-47. doi: 10.1016/j.clinthera.2008.08.008. Clin Ther. 2008. PMID: 18803986 Review.
Cited by
-
Evaluation of activity and combination strategies with the microtubule-targeting drug sagopilone in breast cancer cell lines.Front Oncol. 2011 Nov 16;1:44. doi: 10.3389/fonc.2011.00044. eCollection 2011. Front Oncol. 2011. PMID: 22649765 Free PMC article.
-
Niche-Based Screening in Multiple Myeloma Identifies a Kinesin-5 Inhibitor with Improved Selectivity over Hematopoietic Progenitors.Cell Rep. 2015 Feb 10;10(5):755-770. doi: 10.1016/j.celrep.2015.01.017. Epub 2015 Feb 5. Cell Rep. 2015. PMID: 25660025 Free PMC article.
-
Metal-Dependent Cytotoxic and Kinesin Spindle Protein Inhibitory Activity of Ru, Os, Rh, and Ir Half-Sandwich Complexes of Ispinesib-Derived Ligands.Inorg Chem. 2020 Oct 19;59(20):14879-14890. doi: 10.1021/acs.inorgchem.0c00957. Epub 2020 Oct 1. Inorg Chem. 2020. PMID: 33003697 Free PMC article.
-
Targeting mitotic exit with hyperthermia or APC/C inhibition to increase paclitaxel efficacy.Cell Cycle. 2013 Aug 15;12(16):2598-607. doi: 10.4161/cc.25591. Epub 2013 Jul 9. Cell Cycle. 2013. PMID: 23907120 Free PMC article.
-
A Comprehensive Study on the Electrostatic Properties of Tubulin-Tubulin Complexes in Microtubules.Cells. 2023 Jan 5;12(2):238. doi: 10.3390/cells12020238. Cells. 2023. PMID: 36672172 Free PMC article.
References
-
- Bishop JF, Dewar J, Toner GC, et al. Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol. 1999;17:2355–64. - PubMed
-
- Nabholtz JM, Riva A. Taxane/anthracycline combinations: setting a new standard in breast cancer? Oncologist. 2001;6 (Suppl 3):5–12. - PubMed
-
- Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20:1–15. - PubMed
-
- Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107–17. - PubMed
-
- Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

