Identification of markers of taxane sensitivity using proteomic and genomic analyses of breast tumors from patients receiving neoadjuvant paclitaxel and radiation
- PMID: 20068102
- PMCID: PMC2892225
- DOI: 10.1158/1078-0432.CCR-09-1091
Identification of markers of taxane sensitivity using proteomic and genomic analyses of breast tumors from patients receiving neoadjuvant paclitaxel and radiation
Abstract
Purpose: To identify molecular markers of pathologic response to neoadjuvant paclitaxel/radiation treatment, protein and gene expression profiling were done on pretreatment biopsies.
Experimental design: Patients with high-risk, operable breast cancer were treated with three cycles of paclitaxel followed by concurrent paclitaxel/radiation. Tumor tissue from pretreatment biopsies was obtained from 19 of the 38 patients enrolled in the study. Protein and gene expression profiling were done on serial sections of the biopsies from patients that achieved a pathologic complete response (pCR) and compared to those with residual disease, non-pCR (NR).
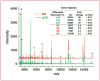
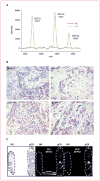
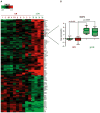
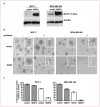
Results: Proteomic and validation immunohistochemical analyses revealed that alpha-defensins (DEFA) were overexpressed in tumors from patients with a pCR. Gene expression analysis revealed that MAP2, a microtubule-associated protein, had significantly higher levels of expression in patients achieving a pCR. Elevation of MAP2 in breast cancer cell lines led to increased paclitaxel sensitivity. Furthermore, expression of genes that are associated with the basal-like, triple-negative phenotype were enriched in tumors from patients with a pCR. Analysis of a larger panel of tumors from patients receiving presurgical taxane-based treatment showed that DEFA and MAP2 expression as well as histologic features of inflammation were all statistically associated with response to therapy at the time of surgery.
Conclusion: We show the utility of molecular profiling of pretreatment biopsies to discover markers of response. Our results suggest the potential use of immune signaling molecules such as DEFA as well as MAP2, a microtubule-associated protein, as tumor markers that associate with response to neoadjuvant taxane-based therapy.
Conflict of interest statement
A.B. Chakravarthy: commercial research grants for the support of the clinical trials from Aventis and BMS. The other authors disclosed no potential conflicts of interest.
Figures
Similar articles
-
Functional genomics identifies ABCC3 as a mediator of taxane resistance in HER2-amplified breast cancer.Cancer Res. 2008 Jul 1;68(13):5380-9. doi: 10.1158/0008-5472.CAN-08-0234. Cancer Res. 2008. PMID: 18593940
-
The determination of changes in the expression of genes for selected specific transcriptional factors in in vitro ductal breast cancer cells under the influence of paclitaxel.Cell Mol Biol Lett. 2011 Dec;16(4):610-24. doi: 10.2478/s11658-011-0026-8. Epub 2011 Sep 7. Cell Mol Biol Lett. 2011. PMID: 21909792 Free PMC article.
-
Specific kinesin expression profiles associated with taxane resistance in basal-like breast cancer.Breast Cancer Res Treat. 2012 Feb;131(3):849-58. doi: 10.1007/s10549-011-1500-8. Epub 2011 Apr 9. Breast Cancer Res Treat. 2012. PMID: 21479552 Free PMC article.
-
Emerging role of taxanes in adjuvant and neoadjuvant therapy for breast cancer: the potential and the questions.Surg Clin North Am. 2003 Aug;83(4):943-71. doi: 10.1016/S0039-6109(03)00071-9. Surg Clin North Am. 2003. PMID: 12875604 Review.
-
Evidence-based use of neoadjuvant taxane in operable and inoperable breast cancer.Clin Cancer Res. 2004 May 15;10(10):3249-61. doi: 10.1158/1078-0432.CCR-03-0133. Clin Cancer Res. 2004. PMID: 15161677 Review.
Cited by
-
Neoadjuvant doxorubicin/cyclophosphamide followed by ixabepilone or paclitaxel in early stage breast cancer and evaluation of βIII-tubulin expression as a predictive marker.Oncologist. 2013;18(7):787-94. doi: 10.1634/theoncologist.2013-0075. Epub 2013 Jul 12. Oncologist. 2013. PMID: 23853246 Free PMC article. Clinical Trial.
-
MALDI Imaging mass spectrometry: current frontiers and perspectives in pathology research and practice.Lab Invest. 2015 Apr;95(4):422-31. doi: 10.1038/labinvest.2014.156. Epub 2015 Jan 26. Lab Invest. 2015. PMID: 25621874 Review.
-
Preoperative concurrent paclitaxel-radiation in locally advanced breast cancer: pathologic response correlates with five-year overall survival.Breast Cancer Res Treat. 2010 Dec;124(3):723-32. doi: 10.1007/s10549-010-1181-8. Epub 2010 Sep 29. Breast Cancer Res Treat. 2010. PMID: 20878462 Free PMC article. Clinical Trial.
-
Myeloid Cell Leukemia 1 Small Molecule Inhibitor S63845 Synergizes with Cisplatin in Triple-Negative Breast Cancer.Cancers (Basel). 2023 Sep 8;15(18):4481. doi: 10.3390/cancers15184481. Cancers (Basel). 2023. PMID: 37760451 Free PMC article.
-
MALDI imaging mass spectrometry for direct tissue analysis: technological advancements and recent applications.Histochem Cell Biol. 2011 Sep;136(3):227-44. doi: 10.1007/s00418-011-0843-x. Epub 2011 Jul 30. Histochem Cell Biol. 2011. PMID: 21805154 Review.
References
-
- Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–74. - PubMed
-
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85. - PubMed
-
- Kaufmann M, von Minckwitz G, Smith R, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003;21:2600–8. - PubMed
-
- Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5R01 GM58008-09/GM/NIGMS NIH HHS/United States
- T32 CA009385/CA/NCI NIH HHS/United States
- R01 CA105436/CA/NCI NIH HHS/United States
- CA070856/CA/NCI NIH HHS/United States
- P50 CA95131/CA/NCI NIH HHS/United States
- R01 CA070856/CA/NCI NIH HHS/United States
- P50 CA098131/CA/NCI NIH HHS/United States
- CA009385/CA/NCI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- ES00267/ES/NIEHS NIH HHS/United States
- P30 ES000267/ES/NIEHS NIH HHS/United States
- CA105436/CA/NCI NIH HHS/United States
- F32 CA138106/CA/NCI NIH HHS/United States
- R01 GM058008/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

