Glioma-associated cancer-initiating cells induce immunosuppression
- PMID: 20068105
- PMCID: PMC2943842
- DOI: 10.1158/1078-0432.CCR-09-1983
Glioma-associated cancer-initiating cells induce immunosuppression
Retraction in
-
Retraction: Glioma-associated cancer-initiating cells induce immunosuppression.Clin Cancer Res. 2015 May 1;21(9):2189. doi: 10.1158/1078-0432.CCR-15-0266. Clin Cancer Res. 2015. PMID: 25934892 Free PMC article. No abstract available.
Abstract
Purpose: Glioblastoma multiforme is a lethal cancer that responds poorly to therapy. Glioblastoma multiforme cancer-initiating cells have been shown to mediate resistance to both chemotherapy and radiation; however, it is unknown to what extent these cells contribute to the profound immunosuppression in glioblastoma multiforme patients and if strategies that alter their differentiation state can reduce this immunosuppression.
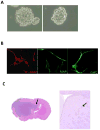
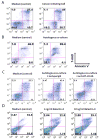
Experimental design: We isolated a subpopulation of cells from glioblastoma multiforme that possessed the capacity for self-renewal, formed neurospheres in vitro, were capable of pluripotent differentiation, and could initiate tumors in vivo. The immune phenotype of these cells was characterized including the elaboration of immunosuppressive cytokines and chemokines by ELISA. Functional immunosuppressive properties were characterized based on the inhibition of T-cell proliferation and effector responses, triggering of T-cell apoptosis, and induction of FoxP3(+) regulatory T cells. On altering their differentiation state, the immunosuppressive phenotype and functional assays were reevaluated.
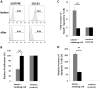
Results: We found that the cancer-initiating cells markedly inhibited T-cell proliferation and activation, induced regulatory T cells, and triggered T-cell apoptosis that was mediated by B7-H1 and soluble Galectin-3. These immunosuppressive properties were diminished on altering the differentiation of the cancer-initiating cells.
Conclusion: Cancer-initiating cells contribute to tumor evasion of the immunosurveillance and approaches that alter the differentiation state may have immunotherapeutic potential.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

