Cancer chemopreventive activity and metabolism of isoliquiritigenin, a compound found in licorice
- PMID: 20068129
- PMCID: PMC2818593
- DOI: 10.1158/1940-6207.CAPR-09-0049
Cancer chemopreventive activity and metabolism of isoliquiritigenin, a compound found in licorice
Abstract
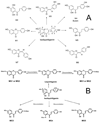
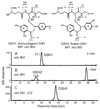
Isoliquiritigenin (2',4',4-trihydroxychalcone; ILG), a chalcone found in licorice root and many other plants, has shown potential chemopreventive activity through induction of phase II enzymes such as quinone reductase-1 in murine hepatoma cells. In this study, the in vivo metabolism of ILG was investigated in rats. In addition, ILG glucuronides and ILG-glutathione adducts were observed in human hepatocytes and in livers from rats treated with ILG. ILG glucuronides were detected in both plasma and rat liver tissues. In addition, in a full-term cancer chemoprevention study conducted with 7,12-dimethylbenz(a)anthracene-treated female Sprague-Dawley rats, dietary administration of ILG slightly increased tumor latency but had a negative effect on the incidence of mammary tumors starting at approximately 65 days after 7,12-dimethylbenz(a)anthracene administration. Further, no significant induction of phase II enzymes was found in mammary glands, which is consistent with the low level of ILG observed in these tissues. However, ILG significantly induced quinone reductase-1 activity in the colon, and glutathione as well as glutathione S-transferase in the liver. Analysis of mRNA expression in tissues of rats treated with ILG supported these findings. These results suggest that ILG should be tested for chemopreventive efficacy in nonmammary models of cancer.
Figures
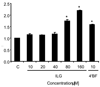



 ). Induction was calculated by comparing group 2 (DMBA and 7.5 g/kg diet of ILG) and group 3 (DMBA and 10.0 g/kg diet of ILG) with group 1 (DMBA in sesame oil), and group 4 (10.0 g/kg diet of ILG) with group 5 (basal diet). Treatment groups were significantly different (p<0.05) from the DMBA only control group 1 (*) or from the basal diet control group 5 (**) with n = 6.
). Induction was calculated by comparing group 2 (DMBA and 7.5 g/kg diet of ILG) and group 3 (DMBA and 10.0 g/kg diet of ILG) with group 1 (DMBA in sesame oil), and group 4 (10.0 g/kg diet of ILG) with group 5 (basal diet). Treatment groups were significantly different (p<0.05) from the DMBA only control group 1 (*) or from the basal diet control group 5 (**) with n = 6.References
-
- Kang Y, Pezzuto J. Induction of quinone reductase as a primary screen for natural product anticarcinogens. Methods Enzymol. 2004;382:380–414. - PubMed
-
- Cao Y, Wang Y, Ji C, Ye J. Determination of liquiritigenin and isoliquiritigenin in Glycyrrhiza uralensis and its medicinal preparations by capillary electrophoresis with electrochemical detection. J Chromatogr A. 2004;1042:203–209. - PubMed
-
- Ramadan M, Kamel M, Ohtani K, Kasai R, Yamasaki K. Minor phenolics from Crinum bulbispermum bulbs. Phytochemistry. 2000;54:891–896. - PubMed
-
- Pan X, Kong L, Zhang Y, Cheng C, Tan R. In vitro inhibition of rat monoamine oxidase by liquiritigenin and isoliquiritigenin isolated from Sinofranchetia chinensis. Acta Pharmacol Sin. 2000;21:949–953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

