Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy
- PMID: 20068137
- PMCID: PMC2844813
- DOI: 10.2337/db09-1336
Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy
Abstract
Objective: Cannabinoid receptor 1 (CB1) is localized in the central nervous system and in peripheral tissues involved in energy metabolism control. However, CB1 receptors are also expressed at low level within the glomeruli, and the aim of this study was to investigate their potential relevance in the pathogenesis of proteinuria in experimental type 1 diabetes.
Research design and methods: Streptozotocin-induced diabetic mice were treated with N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,3-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251), a selective CB1-receptor antagonist, at the dosage of 1 mg x kg(-1) x day(-1) via intraperitoneal injection for 14 weeks. Urinary albumin excretion was measured by enzyme-linked immunosorbent assay. CB1 receptor expression was studied by immunohistochemistry, immunoblotting, and real-time PCR. Expression of nephrin, podocin, synaptopodin, and zonula occludens-1 (ZO-1) was assessed by immunofluorescence and real-time PCR. Fibronectin, transforming growth factor-beta1 (TGF-beta1), and connective tissue growth factor (CTGF) mRNA levels were quantitated by real-time PCR.
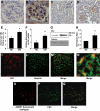
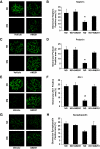
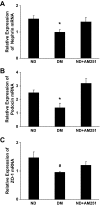
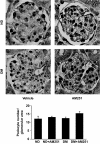
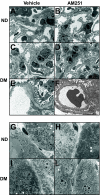
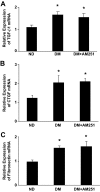
Results: In diabetic mice, the CB1 receptor was overexpressed within the glomeruli, predominantly by glomerular podocytes. Blockade of the CB1 receptor did not affect body weight, blood glucose, and blood pressure levels in either diabetic or control mice. Albuminuria was increased in diabetic mice compared with control animals and was significantly ameliorated by treatment with AM251. Furthermore, CB1 blockade completely prevented diabetes-induced downregulation of nephrin, podocin, and ZO-1. By contrast overexpression of fibronectin, TGF-beta1, and CTGF in renal cortex of diabetic mice was unaltered by AM251 administration.
Conclusions: In experimental type 1 diabetes, the CB1 receptor is overexpressed by glomerular podocytes, and blockade of the CB1 receptor ameliorates albuminuria possibly via prevention of nephrin, podocin, and ZO-1 loss.
Figures
Similar articles
-
Reversal of albuminuria by combined AM6545 and perindopril therapy in experimental diabetic nephropathy.Br J Pharmacol. 2018 Dec;175(23):4371-4385. doi: 10.1111/bph.14495. Epub 2018 Nov 6. Br J Pharmacol. 2018. PMID: 30184259 Free PMC article.
-
Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes.Diabetes. 2009 Sep;58(9):2109-18. doi: 10.2337/db08-0895. Epub 2009 Jul 8. Diabetes. 2009. PMID: 19587356 Free PMC article.
-
Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy.Diabetes. 2011 Sep;60(9):2386-96. doi: 10.2337/db10-1809. Epub 2011 Aug 1. Diabetes. 2011. PMID: 21810593 Free PMC article.
-
Selective phosphodiesterase-5 (PDE-5) inhibitor vardenafil ameliorates renal damage in type 1 diabetic rats by restoring cyclic 3',5' guanosine monophosphate (cGMP) level in podocytes.Nephrol Dial Transplant. 2013 Jul;28(7):1751-61. doi: 10.1093/ndt/gfs391. Epub 2012 Nov 29. Nephrol Dial Transplant. 2013. PMID: 23203993
-
Temporal expression profile and distribution pattern indicate a role of connective tissue growth factor (CTGF/CCN-2) in diabetic nephropathy in mice.Am J Physiol Renal Physiol. 2006 Jun;290(6):F1344-54. doi: 10.1152/ajprenal.00174.2005. Epub 2005 Dec 27. Am J Physiol Renal Physiol. 2006. PMID: 16380465
Cited by
-
The intervention of cannabinoid receptor in chronic and acute kidney disease animal models: a systematic review and meta-analysis.Diabetol Metab Syndr. 2024 Feb 15;16(1):45. doi: 10.1186/s13098-024-01283-2. Diabetol Metab Syndr. 2024. PMID: 38360685 Free PMC article. Review.
-
Blockade of CB1 or Activation of CB2 Cannabinoid Receptors Is Differentially Efficacious in the Treatment of the Early Pathological Events in Streptozotocin-Induced Diabetic Rats.Int J Mol Sci. 2022 Dec 23;24(1):240. doi: 10.3390/ijms24010240. Int J Mol Sci. 2022. PMID: 36613692 Free PMC article.
-
Multiple endocannabinoid-mediated mechanisms in the regulation of energy homeostasis in brain and peripheral tissues.Cell Mol Life Sci. 2019 Apr;76(7):1341-1363. doi: 10.1007/s00018-018-2994-6. Epub 2019 Jan 1. Cell Mol Life Sci. 2019. PMID: 30599065 Free PMC article. Review.
-
The novel, orally available and peripherally restricted selective cannabinoid CB2 receptor agonist LEI-101 prevents cisplatin-induced nephrotoxicity.Br J Pharmacol. 2016 Feb;173(3):446-58. doi: 10.1111/bph.13338. Epub 2016 Jan 15. Br J Pharmacol. 2016. PMID: 26398481 Free PMC article.
-
Reversal of albuminuria by combined AM6545 and perindopril therapy in experimental diabetic nephropathy.Br J Pharmacol. 2018 Dec;175(23):4371-4385. doi: 10.1111/bph.14495. Epub 2018 Nov 6. Br J Pharmacol. 2018. PMID: 30184259 Free PMC article.
References
-
- Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW: American Diabetes Association Nephropathy in diabetes. Diabetes Care 2004; 27: S79– S83 - PubMed
-
- Li JJ, Kwak SJ, Jung DS, Kim JJ, Yoo TH, Ryu DR, Han SH, Choi HY, Lee JE, Moon SJ, Kim DK, Han DS, Kang SW: Podocyte biology in diabetic nephropathy. Kidney Int 2007; 106: S36– S42 - PubMed
-
- Wolf G, Chen S, Ziyadeh FN: From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 2005; 54: 1626– 1634 - PubMed
-
- Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 2003; 83: 253– 307 - PubMed
-
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1998; 1: 572– 578 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

