Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes
- PMID: 20068167
- PMCID: PMC2817492
- DOI: 10.1158/0008-5472.CAN-09-1688
Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes
Abstract
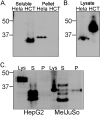
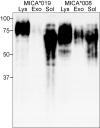
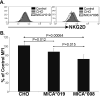
The MHC class I-related chain (MIC) A and MICB ligands for the activating receptor NKG2D can be shed from tumor cells, and the presence of these soluble molecules in sera is related with compromised immune response and progression of disease. Recently, thiol disulphide isomerases and members of the ADAM (a disintegrin and metalloproteinase) gene family were identified as key enzymes in mediating MICA/B shedding from cells. Here, we report shedding of the most frequently expressed MICA allele in human populations (MICA*008) into exosomes, small membrane vesicles that are secreted upon fusion with the plasma membrane. Although similar to other MICA/B molecules in the extracellular domain, the predicted transmembrane and cytoplasmic domains of MICA*008 are quite different, and this difference seemed to be critical for the mode of release from tumor cells. Treatment of natural killer (NK) cells with exosomes containing MICA*008 molecules not only triggered downregulation of NKG2D from the cell surface but also provoked a marked reduction in NK cytotoxicity that is independent of NKG2D ligand expression by the target cell. Our findings reveal a mechanism of NK suppression in cancer that may facilitate immune escape and progression.
Figures



References
-
- Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress- inducible MICA. Science. 1999;285:727–9. - PubMed
-
- Maasho K, Opoku-Anane J, Marusina AI, Coligan JE, Borrego F. NKG2D is a costimulatory receptor for human naive CD8+ T cells. J Immunol. 2005;174:4480–4. - PubMed
-
- Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–72. - PubMed
-
- Gonzalez S, Groh V, Spies T. Immunobiology of human NKG2D and its ligands. Curr Top Microbiol Immunol. 2006;298:121–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

