Modulation of T-cell activation by malignant melanoma initiating cells
- PMID: 20068175
- PMCID: PMC2883769
- DOI: 10.1158/0008-5472.CAN-09-1592
Modulation of T-cell activation by malignant melanoma initiating cells
Abstract
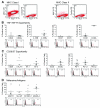
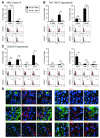
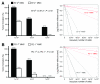
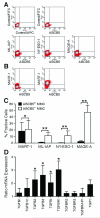
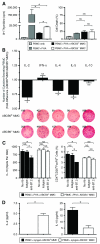
Highly immunogenic cancers such as malignant melanoma are capable of inexorable tumor growth despite the presence of antitumor immunity. Thus, only a restricted minority of tumorigenic malignant cells may possess the phenotypic and functional characteristics needed to modulate tumor-directed immune activation. Here we provide evidence supporting this hypothesis. Tumorigenic ABCB5(+) malignant melanoma initiating cells (MMICs) possessed the capacity to preferentially inhibit IL-2-dependent T-cell activation and to support, in a B7.2-dependent manner, induction of CD4(+)CD25(+)FoxP3(+) regulatory T cells (Tregs). Compared with melanoma bulk cell populations, ABCB5(+) MMICs displayed lower levels of MHC class I, aberrant positivity for MHC class II, and lower expression levels of the melanoma-associated antigens MART-1, ML-IAP, NY-ESO-1, and MAGE-A. Additionally, these tumorigenic ABCB5(+) subpopulations preferentially expressed the costimulatory molecules B7.2 and PD-1, both in established melanoma xenografts and in clinical tumor specimens. In immune activation assays, MMICs inhibited mitogen-dependent human peripheral blood mononuclear cell (PBMC) proliferation and IL-2 production more efficiently than ABCB5(-) melanoma cell populations. Moreover, coculture with ABCB5(+) MMICs increased the abundance of Tregs, in a B7.2 signaling-dependent manner, along with IL-10 production by mitogen-activated PBMCs. Consistent with these findings, MMICs also preferentially inhibited IL-2 production and induced IL-10 secretion by cocultured patient-derived, syngeneic PBMCs. Our findings identify novel T-cell modulatory functions of ABCB5(+) melanoma subpopulations and suggest specific roles for these MMICs in the evasion of antitumor immunity and in cancer immunotherapeutic resistance.
Figures

Similar articles
-
Identification of cells initiating human melanomas.Nature. 2008 Jan 17;451(7176):345-9. doi: 10.1038/nature06489. Nature. 2008. PMID: 18202660 Free PMC article.
-
The helicase HAGE prevents interferon-α-induced PML expression in ABCB5+ malignant melanoma-initiating cells by promoting the expression of SOCS1.Cell Death Dis. 2014 Feb 13;5(2):e1061. doi: 10.1038/cddis.2014.29. Cell Death Dis. 2014. PMID: 24525737 Free PMC article.
-
VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth.Cancer Res. 2011 Feb 15;71(4):1474-85. doi: 10.1158/0008-5472.CAN-10-1660. Epub 2011 Jan 6. Cancer Res. 2011. PMID: 21212411 Free PMC article.
-
Tumor initiation in human malignant melanoma and potential cancer therapies.Anticancer Agents Med Chem. 2010 Feb;10(2):131-6. doi: 10.2174/187152010790909254. Anticancer Agents Med Chem. 2010. PMID: 20184545 Free PMC article. Review.
-
Melanoma stem cells and metastasis: mimicking hematopoietic cell trafficking?Lab Invest. 2014 Jan;94(1):13-30. doi: 10.1038/labinvest.2013.116. Epub 2013 Oct 14. Lab Invest. 2014. PMID: 24126889 Free PMC article. Review.
Cited by
-
Immune evasion by cancer stem cells.Regen Ther. 2021 Mar 11;17:20-33. doi: 10.1016/j.reth.2021.02.006. eCollection 2021 Jun. Regen Ther. 2021. PMID: 33778133 Free PMC article. Review.
-
Phenotypic, functional, and metabolic heterogeneity of immune cells infiltrating non-small cell lung cancer.Front Immunol. 2022 Aug 10;13:959114. doi: 10.3389/fimmu.2022.959114. eCollection 2022. Front Immunol. 2022. PMID: 36032082 Free PMC article. Review.
-
Tumor heterogeneity, clonal evolution, and therapy resistance: an opportunity for multitargeting therapy.Discov Med. 2013 Mar;15(82):188-94. Discov Med. 2013. PMID: 23545047 Free PMC article.
-
Melanocytes, melanocyte stem cells, and melanoma stem cells.Clin Dermatol. 2013 Mar-Apr;31(2):166-78. doi: 10.1016/j.clindermatol.2012.08.014. Clin Dermatol. 2013. PMID: 23438380 Free PMC article. Review.
-
Genetically determined ABCB5 functionality correlates with pigmentation phenotype and melanoma risk.Biochem Biophys Res Commun. 2013 Jul 5;436(3):536-42. doi: 10.1016/j.bbrc.2013.06.006. Epub 2013 Jun 12. Biochem Biophys Res Commun. 2013. PMID: 23770371 Free PMC article.
References
-
- Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

